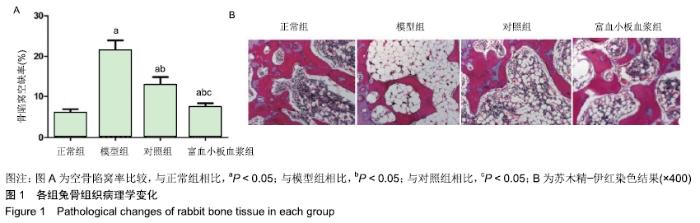
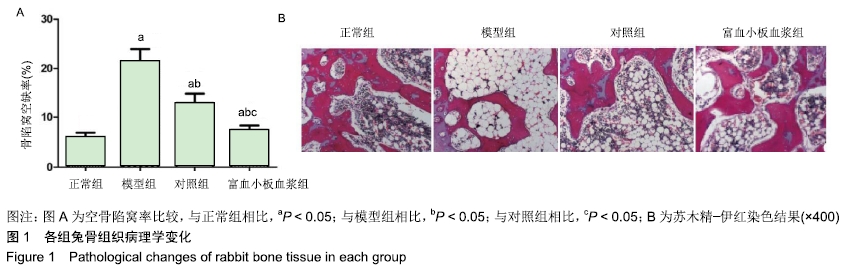
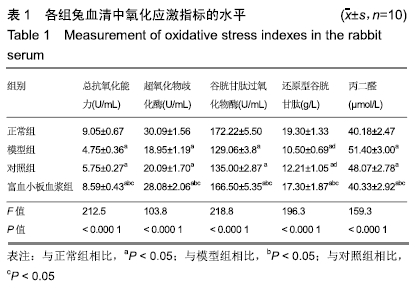
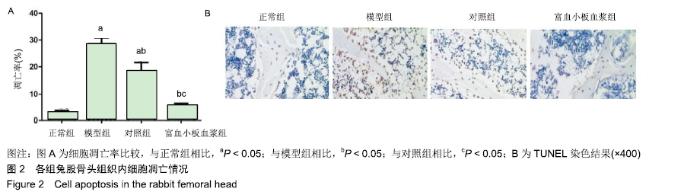
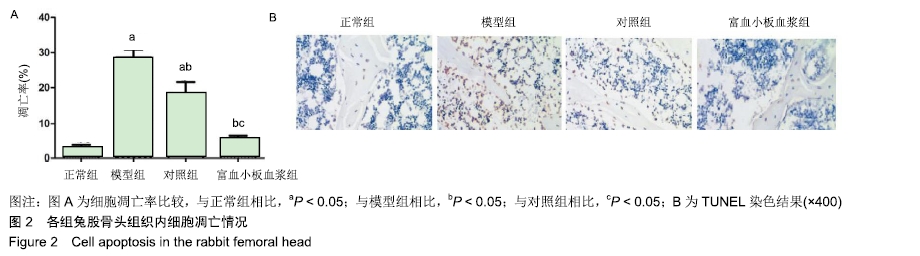
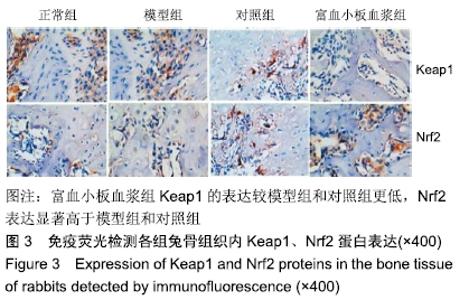
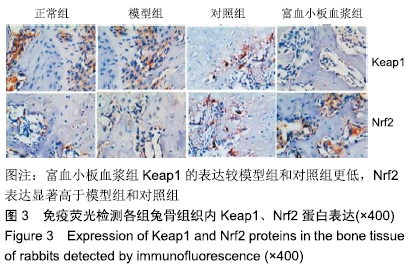
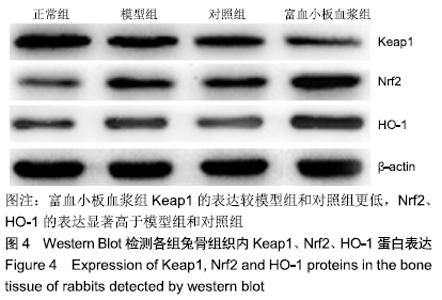
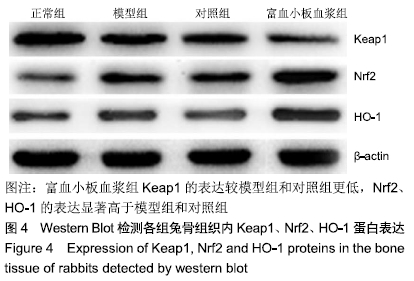
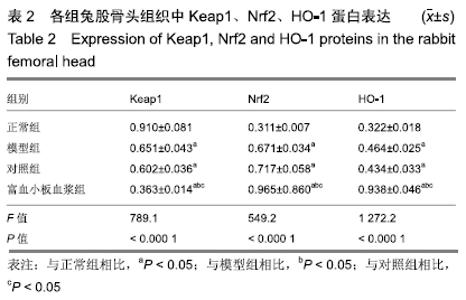
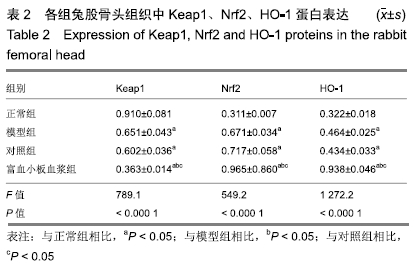
[1] 刘鑫,刘秋亮,王秀利,等.关节囊减压、坏死骨刮除和植骨治疗儿童股骨头坏死7例[J]. 郑州大学学报(医学版),2015,50(4):580-582.
[2] 赵德伟,谢辉.成人股骨头坏死保髋手术治疗的策略及探讨[J].中国修复重建外科杂志,2018,32(7):792-797.
[3] 张恩景,廖文,蔡进奎,等.非创伤性股骨头坏死的基因多态性研究进展[J].中国骨质疏松杂志,2018,24(4):543-546+551.
[4] 杨春梅.化痰祛瘀补肾法治疗激素相关性股骨头坏死临床疗效观察[J].中国现代药物应用,2019,13(11):172-173.
[5] LEE YK, LEE B, Parvizi J, et al. Which Osteotomy for Osteonecrosis of the Femoral Head and Which Patient for the Osteotomy?.Clin Orthop Surg. 2019;11(2):137-141.
[6] 农焦,曾平.TLR4/NF-κB信号转导通路在激素性股骨头坏死防治研究中的应用[J].中华中医药学刊,2019,37(7):1689-1693.
[7] 佟鹏,王洋,梁瀛.激素性股骨头缺血性坏死动物模型的建立及综合评估[J].中国组织工程研究,2018,22(32):5169-5174.
[8] 强辉.基于数字医学检测方法研究三七总皂甙对兔早期激素性股骨头坏死的作用及机制[J].中国数字医学,2017,12(11):36-40.
[9] 韩丹丹. 绿原酸对地塞米松诱导小鼠MC3T3-E1成骨细胞凋亡的保护机制[D].中国农业大学,2018.
[10] 安丽平,于琨,耿海波,等.丹酚酸B抗氧化应激损伤机制的研究进展[J].中国实验方剂学杂志,2019,25(22):1-8.
[11] ANTOGNELLI C,TRAPANI E, DELLE MS, et al. KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease.Free Radic Biol Med. 2018;115:202-218.
[12] 李松涛.SC79通过激活Akt-Nrf2信号抑制地塞米松损害成骨细胞的机制研究[D].苏州:苏州大学,2017.
[13] 强辉,王坤正,高培国,等.氧化应激对激素性股骨头缺血坏死微血管的损伤作用[J].西安交通大学学报(医学版), 2009,30(3):352-355.
[14] 牛科润,杨孟恺,马春辉,等.多孔纳米复合材料Se@SiO_2治疗激素性股骨头坏死[J].中国组织工程研究, 2017,21(22):3476-3482.
[15] 刘江锋.髓芯减压联合自体骨髓间充质干细胞移植治疗股骨头坏死[J].中国组织工程研究,2019,23(29):4599-4604.
[16] 赵德伟,谢辉.成人股骨头坏死保髋手术治疗的策略及探讨[J].中国修复重建外科杂志,2018,32(7):792-797.
[17] 杨富强,杨晓明,葛建健,等 髓芯减压植骨联合富血小板血浆治疗股骨头缺血性坏死的前瞻随机对照研究[J]. 中华关节外科杂志(电子版), 2016, 10(2):22-25.
[18] 于鹏,纪志华,贾丙申,等.髓芯减压联合富血小板血浆对兔激素性股骨头坏死的疗效及对金属蛋白酶/基质金属蛋白酶组织抑制剂系统的影响[J].中国比较医学杂志,2018,28(2):53-58.
[19] 翟玥,邓国英,王秋根,等.活性氧与激素性股骨头坏死[J].国际骨科学杂志, 2017,38(2):102-105.
[20] 代志鹏.谷胱甘肽对激素性股骨头坏死骨髓间充质干细胞分化的影响[D].武汉:华中科技大学,2015.
[21] QUERIO G, ANTONIOTTI S, FOGLIETTA F, et al.Chamazulene Attenuates ROS Levels in Bovine Aortic Endothelial Cells Exposed to High Glucose Concentrations and Hydrogen Peroxide. Front Physiol.2018;9:246.
[22] AN J, ZHOU Q, WU M, et al.Interactions between oxidative stress, autophagy and apoptosis in A549 cells treated with aged black carbon. Toxicol In Vitro. 2019;54:67-74.
[23] 王亚苹,张振坤,李喆,等.重组人MG53蛋白激活Akt/GSK-3β通路降低LPS诱导的hUC-MSCs氧化损伤[J].郑州大学学报(医学版), 2019, 54(4): 522-526.
[24] CONG P, LIU Y, LIU N, et al.Cold exposure induced oxidative stress and apoptosis in the myocardium by inhibiting the Nrf2-Keap1 signaling pathway.BMC Cardiovasc Disord. 2018;18(1):36.
[25] 张佳婵,程琳,化璟琳,等.沙棘粕醇提取物对小鼠氧化应激损伤的保护作用及机制[J].中国食品学报,2019,(7):11-19.
[26] WU J, SUN B, LUO X, et al.Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. Rsc Advances.2018;8(20):10898-10906.
[27] 于鹏,纪志华,贾丙申,等.髓芯减压联合富血小板血浆对兔激素性股骨头坏死的疗效及对金属蛋白酶/基质金属蛋白酶组织抑制剂系统的影响[J].中国比较医学杂志,2018,28(02):53-58.
[28] 张波,韦冰丹,甘坤宁,等.富血小板血浆联合骨髓间充质干细胞对兔股骨头坏死BMP-2/Smads通路的影响[J].中国骨质疏松杂志, 2016,22(2): 131-134+227.
[29] MARTINS RP, HARTMANN DD, DE MORAES JP, et al.Platelet-rich plasma reduces the oxidative damage determined by a skeletal muscle contusion in rats.Platelets. 2016;27(8):784-790.
[30] SEKERCI CA, TANIDIR Y, SENER TE, et al.Effects of platelet-rich plasma against experimental ischemia/reperfusion injury in rat testis.J Pediatr Urol. 2017;13(3):317.e1-317.e9.
|