Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (28): 4445-4451.doi: 10.3969/j.issn.2095-4344.2292
Previous Articles Next Articles
How does extracellular matrix-based growth factor delivery system promote osteogenesis?
Shi Jun1, Yang Lin1, Guo Zhibin1, Cui Yutao2, Liu He2
1Second Department of Orthopedics, Xiehe Dongxihu Hospital/Dongxihu District People’s Hospital, Wuhan 430040, Hubei Province, China; 2Department of Orthopedics, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China
-
Received:2019-11-16Revised:2019-11-20Accepted:2019-12-20Online:2020-10-08Published:2020-08-31 -
Contact:Liu He, MD, Attending physician, Department of Orthopedics, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China -
About author:Shi Jun, Master, Second Department of Orthopedics, Xiehe Dongxihu Hospital/Dongxihu District People’s Hospital, Wuhan 430040, Hubei Province, China -
Supported by:A Project of Department of Finance of Jinlin Province, No. 2019SCZT014
CLC Number:
Cite this article
Shi Jun, Yang Lin, Guo Zhibin, Cui Yutao, Liu He. How does extracellular matrix-based growth factor delivery system promote osteogenesis? [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4445-4451.
share this article
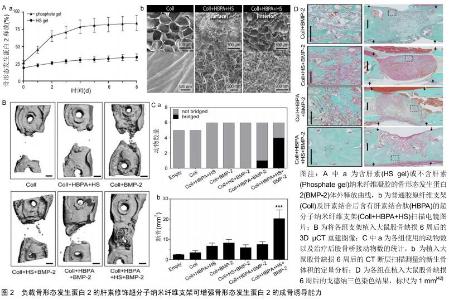
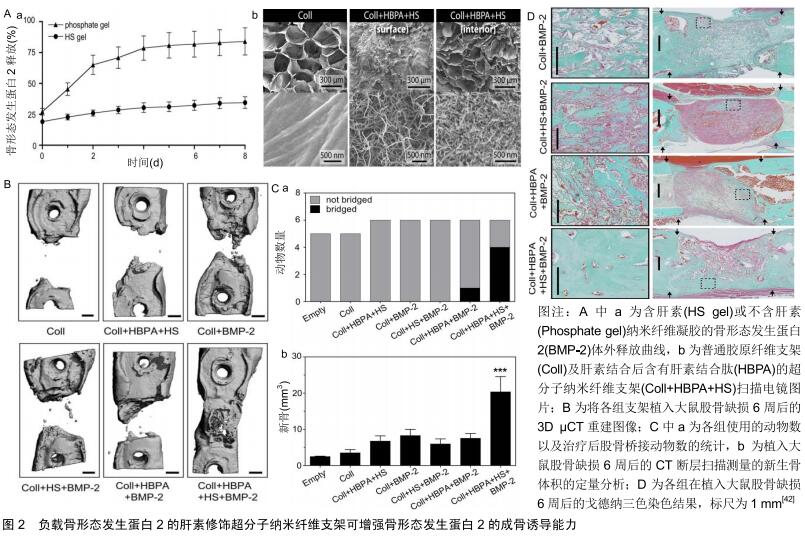
2.1 细胞外基质对生长因子的作用 在人体内环境中,细胞外基质在协调生长因子信号传递方面起着基础性作用,其可以在特定的时间及部位控制性释放生长因子,调节生长因子与细胞之间的信号传导[24]。因此,首先了解细胞外基质如何调节生长因子的活性,将会是提升生长因子局部递送系统的关键[25]。 细胞外基质并不仅仅是一种纤维支持结构,它还是一种不断动态变化的微环境,可调控多种细胞功能。细胞外基质为细胞迁移等提供了一个支架结构,细胞外基质中的胶原蛋白和糖蛋白为黏附受体(如整合素)等提供了许多细胞结合位点[26]。整合素是一种重要的跨膜细胞表面受体,其可识别多种细胞外基质蛋白(如胶原蛋白、纤连蛋白等)中的短序列,使细胞与细胞外基质之间产生相互作用[27]。同时整合素相关的信号途径可以调控细胞增殖分化等多种的细胞功能,更重要的是细胞外基质是生长因子的储存库,因为它有着大量的生长因子结合位点[28-29]。骨形态发生蛋白2、血管内皮生长因子等多种生长因子可以与细胞外基质中的硫酸肝素蛋白多糖特异性的结合,称为肝素结合性生长因子[30-31]。此外细胞外基质中的纤连蛋白[32]、纤维蛋白原[33]、肌腱蛋白C和玻连蛋白等均是骨再生过程中的重要的生长因子的结合位点[34-35]。生长因子结合于特定位点后的释放,主要取决于生长因子与结合蛋白之间的亲和力及蛋白酶的作用[36]。因此在骨缺损等局部微环境中,与细胞外基质结合的生长因子可在特定的时间、位置以特定的方式释放,调控细胞的增殖分化,在骨再生过程的不同阶段释放大量生长因子,可以临时与细胞外基质内的特定位点结合。因此,生长因子在找到它们的同源细胞表面受体之前将首先与基质成分相互作用。此外,生长因子与细胞外基质组分间形成的分子复合物可调控生长因子受体的信号传导,影响生长因子的作用效果[37-38]。 2.2 细胞外基质性支架 生长因子递送系统旨在骨缺损局部缓慢释放各类生长因子,保持局部适当浓度的活性物质,降低对正常组织的影响,发挥最佳的促成骨作用,而这也与生理状态下细胞外基质的功能相似。因此将细胞质外基质与生长因子间的这些联系整合到生长因子递送系统中,能够极大提高生长因子的活性和治疗效果。 2.2.1 支架的肝素化修饰 近年来多种天然及人工合成的材料被成功应用于骨组织工程,如壳聚糖、纤维蛋白凝胶、Ⅰ型胶原、聚己内酯、聚乳酸-羟基乙酸共聚物等。这些材料通过表面修饰、生物涂层、制作混合物等方式可成功负载生长因子形成复合系统用于骨缺损局部,其在动物实验研究中展现出了良好的促进成骨的作用[39]。但是在这些复合系统中,生长因子的释放主要取决于其与支架本身的相互作用,如疏水性和亲水性作用、化学键或静电吸引力的消失等,这些作用是不够精确可控的[40-41]。 由于细胞外基质与细胞因子间具有很好的亲和力,将细胞外基质中的这些结合分子提取出来整合入支架材料中,可为细胞因子的负载提供一个确切的结合位点。硫酸肝素蛋白多糖是细胞外基质中结合生长因子的重要组成部分,多种生长因子如骨形态发生蛋白、成纤维细胞生长因子等可与其特异性的结合。因此用肝素来修饰支架材料后可提升生长因子与支架的结合能力以及释放能力。LEE等[42]以具有蛋白多糖及纤维蛋白功能的超分子纳米纤维为基础,将一种双亲性的多肽片段整合入纤维孔隙内从而结合肝素形成复合支架。该复合支架负载骨形态发生蛋白2后成骨能力明显高于普通支架,并且其骨形态发生蛋白2用量明显低于一般用量一个数量级(图2)。YANG等[43]将肝素结合的纤维蛋白原与凝血酶混合后制成了可注射的肝素结合纤维蛋白凝胶,将凝胶负载骨形态发生蛋白2后研究发现,肝素结合纤维蛋白在13 d内释放了(89.4±3.8)%的骨形态发生蛋白2,而单纯纤维蛋白凝胶在最初3 d内就释放了(83.7± 7.6)%的骨形态发生蛋白2。将其植入大鼠后肢肌囊8周后,相比于对照组,负载骨形态发生蛋白2的肝素结合纤维蛋白产生了最广泛的骨形成。ZHU等[44]利用阿仑膦酸钠与肝素及磷酸钙之间的较好亲和力制作了负载骨形态发生蛋白2的磷酸钙支架,他们首先将阿仑膦酸钠与肝素结合形成复合物,再将此复合物通过阿仑膦酸钠结合于磷酸钙支架中,最后骨形态发生蛋白2通过肝素负载于磷酸钙支架上。相比于单纯的磷酸钙支架,这一复合支架中骨形态发生蛋白2更加稳定的结合于其表面,并且展现了更加良好的成骨效应。而相似的,相比于普通的纤维蛋白凝胶,由肝素功能化的纳米颗粒和纤维蛋白凝胶组成的功能性纳米颗粒-水凝胶复合物负载骨形态发生蛋白2后,可显著提高骨形态发生蛋白2局部释放系统的成骨能力(图3)[45]。 "
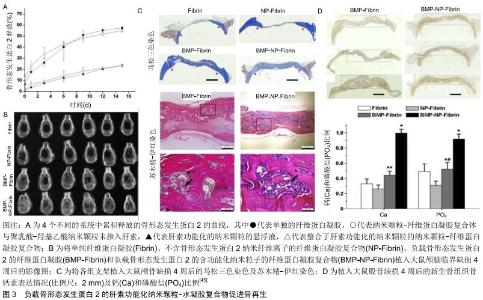
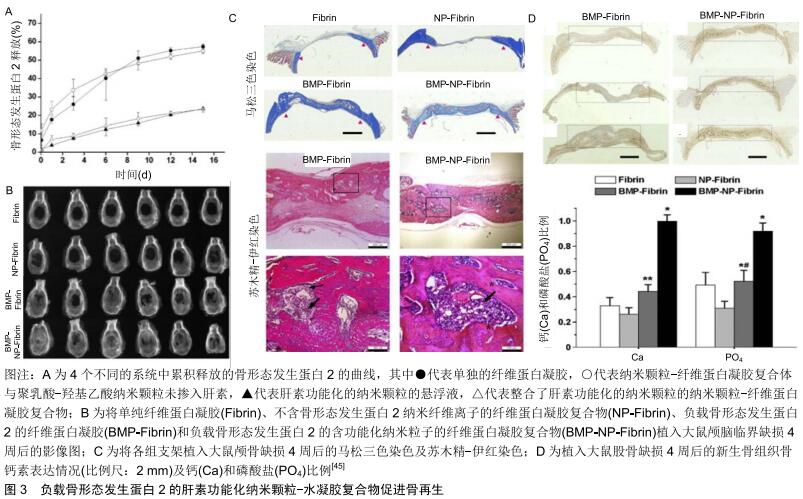
2.2.2 生长因子的结合性修饰 除了修饰生物材料而增强支架与生长因子间的亲和力外,通过修饰生长因子而使其获得良好的材料结合能力也是一种很好的负载策略。许多非特异性的化学交联方法可增强生长因子与载体的结合,但是同时这些化学交联也可能会使的生长因子失去部分功能[25]。为了解决这一问题,将多肽序列整合入生长因子可使得其材料基质交联的同时保留正常的功能。比如SCHMOEKEL等[46]将转谷氨酰胺酶因子XⅢa的底物序列整合入骨形态发生蛋白2中,形成了具有和纤维蛋白结合能力的生长因子。底物结构的存在使得生长因子可以在转谷氨酰胺酶因子XⅢa促进纤维蛋白聚合的过程中成功整合入纤维蛋白基质内,并保持其原有的形态和功能。将这一复合系统植入大鼠临界大小的颅骨缺损内进行评估发现,相比于含有普通骨形态发生蛋白2的纤维蛋白支架,这种具有特殊结合能力的骨形态发生蛋白2能使缺损多愈合约76%。将其应用于犬全腕关节融合术后发现,它比自体松质骨移植能更快地诱导更广泛的骨性连接形成。当生长因子与纤维蛋白基质共价结合后,其释放取决于细胞分泌或细胞活化的蛋白酶(如基质金属蛋白酶和纤溶酶)所介导的基质降解速率,使得生长因子的局部释放更加符合生理状态。 为了使得生长因子释放系统更加符合细胞的实际需要,将酶敏感性的多肽序列整合入生长因子内发挥了较为理想的作用。ARRIGHI等[47]将一个包含N端纤维蛋白结合序列和一个蛋白酶敏感序列的多肽序列整合入甲状旁腺激素的活性片段(parathyroid hormone1-34, PTH1-34)。而PTH1-34的N段修饰抑制了激素的活性,使其在无蛋白酶存在的情况下不表现出生物活性,而在蛋白酶裂解后其N端序列后恢复活性。因此,这种与纤维蛋白结合的PTH1-34只有在细胞需要时才会被激活。在羊的骨再生模型中其显示出剂量依赖性的优良骨形成能力。 2.2.3 生长因子的靶向性修饰 生长因子在缺损局部的释放很大程度上减少了其对正常组织的影响,但是其释放后的分布仍缺乏特异性,因此利用与内源性细胞外基质特异性结合对生长因子进行修饰,可使得生长因子释放到缺损局部后具有特异性的结合能力,而进一步提高其生物活性[36]。在骨形态发生蛋白2的N端添加胶原结合域使其能够特异性结合胶原蛋白。胶原结合域-骨形态发生蛋白2具有良好的生物活性,将负载胶原结合域-骨形态发生蛋白2的脱矿骨基质植入大鼠皮下观察到均匀的骨形成。此外在兔下颌骨缺损模型中,负载胶原结合域-骨形态发生蛋白2的胶原基质植入后表现出较好的骨诱导性能和良好的均匀骨形成[48]。HAN等[49]将胶原结合域整合入骨形态发生蛋白2后将其负载于胶原蛋白支架植入小鼠后外侧脊柱融合术中研究其成骨性能,在术后16周时,含有胶原结合域-骨形态发生蛋白2的支架组脊柱融合达到100%,而普通支架组仅达40%。此外,胶原结合域-骨形态发生蛋白2的支架组具有更好的成骨效果,包括更高的融合效率、更高的骨密度以及更多的骨小梁形成 。 储存在细胞外基质内的肝素结合性生长因子由于细胞外基质的保护作用,其半衰期得以延长,生物利用度可得到极大的提高。因此对非肝素结合性生长因子进行相应的修饰,以提高其在体内对内源性硫酸肝素和糖胺聚糖的亲和力,可提高生长因子在局部释放后的生物利用度,这一概念目前已被应用于软骨组织工程。TOKUNOU等[50]将含有肝素结合域的多肽整合入类胰岛素生长因子1中,合成了可特异性结合肝素的类胰岛素生长因子1。这种改良后的生长因子展现了良好的性能,其在富含蛋白聚糖的环境中保持力明显提高,生物活性持续性明显增强。但是该方法在骨再生中的应用却鲜有报道,但是这一概念的成功实现有望增强生长因子在缺损局部诱导骨再生的能力。 2.2.4 细胞外基质-生长因子复合物 除了与生长因子相结合提高其生物活性以外,细胞外基质还可以直接调控生长因子相关的信号传导。许多生长因子的信号通路是由生长因子、细胞外基质蛋白、黏附受体和生长因子受体之间的动态相互作用调控的[38,51]。生长因子与细胞外基质所形成的复合物,如纤连蛋白和玻连蛋白可影响生长因子的信号传导作用,影响其成骨效应[52-54]。特别是细胞外基质蛋白-生长因子复合物,可使得生长因子受体和整合素之间形成聚合簇。因为生长因子受体和整合素的信号传导机制有许多共同的分子,这些聚合簇的形成增强和延长了信号传递。因此将这一作用整合入生长因子释放系统,可以实现以较低剂量生长因子诱导更显著的成骨效应[55-56]。KISIEL等[57]将包含整合素结合域的结构稳定的纤连蛋白片段整合入透明质酸水凝胶内,作为骨形态发生蛋白2的载体用于骨缺损的研究,发现纤连蛋白的加入增强了透明质酸水凝胶的细胞黏附能力。更重要的是在大鼠异位骨形成模型中与在单纯的透明质酸水凝胶相比,这一负载骨形态发生蛋白2后的复合水凝胶可形成2倍多具有更好胶原纤维组织的新生骨。MARTINO等[58]的研究发现在大鼠临界大小的颅骨缺损模型中,骨形态发生蛋白2和血小板衍生生长因子BB与多功能纤连蛋白片段局部递送系统能够在低剂量(100 ng)下诱导骨再生,而无纤连蛋白片段材料中的相同浓度生长因子没有显著骨再生作用。SHEKARAN 等[59]设计了一种包含α2β1整合素特异性肽(GFOGER)的蛋白酶可降解聚乙二醇合成水凝胶作为骨形态发生蛋白2的载体,该水凝胶可在体内持续释放骨形态发生蛋白2,较低剂量的生长因子显著增加了骨祖细胞在缺损部位的定位并增强了骨形成,诱导了缺损部位的愈合,而相同剂量的骨形态发生蛋白2在胶原海绵递送系统中几乎没有刺激骨骼再生的作用。这些发现表明,GFOGER水凝胶以低的骨形态发生蛋白2剂量促进具有挑战性的缺损的骨再生,是生长因子治疗方法的有效递送载体。 "
|
[1] DIMITRIOU R, JONES E, MCGONAGLE D, et al. Bone regeneration: current concepts and future directions.BMC Med.2011;9:66.
[2] AUDIGE L, GRIFFIN D, BHANDARI M, et al. Path analysis of factors for delayed healing and nonunion in 416 operatively treated tibial shaft fractures. Clin Orthop Relat Res. 2005;438: 221-232.
[3] CALCIOLARI E, MARDAS N, DEREKA X, et al. The effect of experimental osteoporosis on bone regeneration: part 2, proteomics results. Clin Oral Implants Res. 2017;28(9): e135-e45.
[4] ILYAS A, ODATSU T, SHAH A, et al. Amorphous Silica: A New Antioxidant Role for Rapid Critical-Sized Bone Defect Healing. Adv Healthc Mater.2016;5(17):2199-2213.
[5] LI M, WANG W, ZHU Y, et al. Molecular and cellular mechanisms for zoledronic acid-loaded magnesium-strontium alloys to inhibit giant cell tumors of bone. Acta Biomater. 2018; 77:365-379.
[6] LYNCH JR, TAITSMAN LA, BAREI DP, et al. Femoral nonunion: risk factors and treatment options.J Am Acad Orthop Surg. 2008;16(2):88-97.
[7] MARTIN V, BETTENCOURT A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mat Sci Eng C-Mater. 2018;82:363-371.
[8] GRIFFIN KS, DAVIS KM, MCKINLEY TO, et al. Evolution of Bone Grafting: Bone Grafts and Tissue Engineering Strategies for Vascularized Bone Regeneration.Clin Rev Bone Miner. 2015;13(4):232-244.
[9] SOKOLSKY-PAPKOV M, AGASHI K, OLAYE A, et al. Polymer carriers for drug delivery in tissue engineering.Adv Drug Deliver Rev.2007;59(4-5):187-206.
[10] SHRIVATS AR, MCDERMOTT MC, HOLLINGER JO. Bone tissue engineering: state of the union.Drug Discov Today. 2014;19(6):781-786.
[11] LI YH, WANG ZD, WANG W, et al. The biocompatibility of calcium phosphate cements containing alendronate-loaded PLGA microparticles in vitro.Exp Biol Med. 2015;240(11): 1465-1471.
[12] DANG PN, DWIVEDI N, PHILLIPS LM, et al. Controlled Dual Growth Factor Delivery From Microparticles Incorporated Within Human Bone Marrow-Derived Mesenchymal Stem Cell Aggregates for Enhanced Bone Tissue Engineering via Endochondral Ossification. Stem Cells Transl Med. 2016;5(2): 206-217.
[13] AZEVEDO HS, PASHKULEVA I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv Drug Deliv Rev. 2015;94:63-76.
[14] NI M, LI G, TANG PF, et al. rhBMP-2 not alendronate combined with HA-TCP biomaterial and distraction osteogenesis enhance bone formation.Arch Orthop Trauma Surg. 2011;131(11):1469-1476.
[15] WANG C, ZHAO Q, WANG M. Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering.Biofabrication. 2017;9(2):025031.
[16] EVEN J, ESKANDER M, KANG J. Bone morphogenetic protein in spine surgery: current and future uses.J Am Acad Orthop Surg.2012;20(9):547-552.
[17] KITAMURA M, AKAMATSU M, MACHIGASHIRA M, et al. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial.J Dent Res. 2011;90(1): 35-40.
[18] BALOOCH G, BALOOCH M, NALLA RK, et al. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci U S A.2005;102(52):18813-18818.
[19] VOGEL V, SHEETZ M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7(4):265-275.
[20] TRAPPMANN B, GAUTROT JE, CONNELLY JT, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11(7):642-649.
[21] 王之发.细胞外基质支架材料在软骨组织再生和骨组织工程中应用的初步探讨[D].西安:第四军医大学,2016.
[22] HYNES RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216-1219.
[23] AMORIM S, PIRES RA, DA COSTA DS, et al. Interactions between Exogenous FGF-2 and Sulfonic Groups: in Situ Characterization and Impact on the Morphology of Human Adipose-Derived Stem Cells. Langmuir. 2013;29(25):7983-7992.
[24] 池玉磊,卜宪敏,查玉梅,等.骨髓间充质干细胞复合支架材料治疗骨缺损:研究现状及前景展望[J].中国组织工程研究,2019, 23(29): 4749-4756.
[25] MARTINO MM, BRIQUEZ PS, MARUYAMA K, et al. Extracellular matrix-inspired growth factor delivery systems for bone regeneration.Adv Drug Deliver Rev.2015;94:41-52.
[26] 肖统光,张一民,郭维民,等.细胞外基质来源支架在软骨组织工程中的应用[J].中国组织工程研究,2016,38(20):5737-5744.
[27] WU CH, KO JL, PAN HH, et al. Ni-induced TGF-beta signaling promotes VEGF-a secretion via integrin beta3 upregulation.J Cell Physiol.2019;234(12):22093-22102.
[28] MARTINO MM, BRIQUEZ PS, RANGA A, et al. Heparin- binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A.2013;110(12):4563-4568.
[29] DE LAPORTE L, RICE JJ, TORTELLI F, et al. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain.PloS One.2013;8(4):e62076.
[30] MACRI L, SILVERSTEIN D, CLARK RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev.2007;59(13):1366-1381.
[31] NAKATO H, LI JP. Functions of Heparan Sulfate Proteoglycans in Development: Insights From Drosophila Models.Int Rev Cell Mol Biol.2016;325:275-293.
[32] BENTMANN A, KAWELKE N, MOSS D, et al. Circulating fibronectin affects bone matrix, whereas osteoblast fibronectin modulates osteoblast function.J Bone Miner Res. 2010;25(4): 706-715.
[33] VASCONCELOS DM, GONCALVES RM, ALMEIDA CR, et al. Fibrinogen scaffolds with immunomodulatory properties promote in vivo bone regeneration. Biomaterials. 2016;111:163-178.
[34] HAWKINS AG, JULIAN CM, KONZEN S, et al. Microenvironmental Factors Drive Tenascin C and Src Cooperation to Promote Invadopodia Formation in Ewing Sarcoma. Neoplasia. 2019;21(10):1063-1072.
[35] CHO CB, JUNG SY, PARK CY, et al. A Vitronectin-Derived Bioactive Peptide Improves Bone Healing Capacity of SLA Titanium Surfaces. Materials.2019;12(20).pii: E3400.
[36] MARTINO MM, BRIQUEZ PS, GUC E, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science.2014;343(6173):885-888.
[37] KIM SH, TURNBULL J, GUIMOND S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor.J Endocrinol. 2011; 209(2):139-151.
[38] ZHU J, CLARK RAF. Fibronectin at Select Sites Binds Multiple Growth Factors and Enhances their Activity: Expansion of the Collaborative ECM-GF Paradigm.J Invest Dermatol. 2014;134(4):895-901.
[39] FURUYA H, TABATA Y, KANEKO K. Bone regeneration for murine femur fracture by gelatin hydrogels incorporating basic fibroblast growth factor with different release profiles.Tissue Eng Part A.2014;20(9-10):1531-1541.
[40] VO TN, KASPER FK, MIKOS AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev.2012;64(12):1292-1309.
[41] LEE K, SILVA EA, MOONEY DJ. Growth factor delivery- based tissue engineering: general approaches and a review of recent developments.J R Soc Interface.2011;8(55):153-170.
[42] LEE SS, HUANG BJ, KALTZ SR, et al. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials. 2013;34(2): 452-459.
[43] YANG HS, LA WG, BHANG SH, et al. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16(4): 1225-1233.
[44] ZHU JY, HUANG BL, DING S, et al. Tethering of rhBMP-2 upon calcium phosphate cement via alendronate/heparin for localized, sustained and enhanced osteoactivity.Rsc Adv. 2017;7(33):20281-20292.
[45] CHUNG YI, AHN KM, JEON SH, et al. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle- hydrogel complex. J Control Release. 2007;121(1-2):91-99.
[46] SCHMOEKEL HG, WEBER FE, SCHENSE JC, et al. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol Bioeng. 2005;89(3): 253-262.
[47] ARRIGHI I, MARK S, ALVISI M, et al. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials. 2009;30(9):1763-1771.
[48] CHEN B, LIN H, WANG J, et al. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials. 2007;28(6):1027-1035.
[49] HAN X, ZHANG W, GU J, et al. Accelerated postero-lateral spinal fusion by collagen scaffolds modified with engineered collagen-binding human bone morphogenetic protein-2 in rats. PloS One. 2014;9(5):e98480.
[50] TOKUNOU T, MILLER R, PATWARI P, et al. Engineering insulin-like growth factor-1 for local delivery.FASEB J. 2008; 22(6):1886-1893.
[51] MEINEL L, KAPLAN DL. Silk constructs for delivery of musculoskeletal therapeutics.Adv Drug Deliver Rev. 2012; 64(12):1111-1122.
[52] LIN FB, REN XD, PAN Z, et al. Fibronectin Growth Factor-Binding Domains Are Required for Fibroblast Survival. J Invest Dermatol.2011;131(1):84-98.
[53] ANONYMOUS. Fibronectin in human bone tissue remodelling. Virchows Arch. 2005;447(2):434-434.
[54] MIN SK, KANG HK, JUNG SY, et al. A vitronectin-derived peptide reverses ovariectomy-induced bone loss via regulation of osteoblast and osteoclast differentiation.Cell Death Differ. 2018;25(2):268-281.
[55] ALAM N, GOEL HL, ZARIF MJ, et al. The integrin - Growth factor receptor duet. J Cell Physiol. 2007;213(3):649-653.
[56] COMOGLIO PM, BOCCACCIO C, TRUSOLINO L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol.2003;15(5): 565-571.
[57] KISIEL M, MARTINO MM, VENTURA M, et al. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials. 2013;34(3):704-712.
[58] MARTINO MM, TORTELLI F, MOCHIZUKI M, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing.Sci Transl Med. 2011;3(100):100ra89. SHEKARAN A, GARCIA JR, CLARK AY, et al. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35(21):5453-5461 |
| [1] | Wu Xun, Meng Juanhong, Zhang Jianyun, Wang Liang. Concentrated growth factors in the repair of a full-thickness condylar cartilage defect in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1166-1171. |
| [2] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [3] | Zou Gang, Xu Zhi, Liu Ziming, Li Yuwan, Yang Jibin, Jin Ying, Zhang Jun, Ge Zhen, Liu Yi. Human acellular amniotic membrane scaffold promotes ligament differentiation of human amniotic mesenchymal stem cells modified by Scleraxis in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1037-1044. |
| [4] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [5] | Hua Haotian, Zhao Wenyu, Zhang Lei, Bai Wenbo, Wang Xinwei. Meta-analysis of clinical efficacy and safety of antibiotic artificial bone in the treatment of chronic osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 970-976. |
| [6] | Zhang Bin, Sun Lihua, Zhang Junhua, Liu Yusan, Cui Caiyun. A modified flap immediate implant is beneficial to soft tissue reconstruction in maxillary aesthetic area [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 707-712. |
| [7] | Luo Xuanxiang, Jing Li, Pan Bin, Feng Hu. Effect of mecobalamine combined with mouse nerve growth factor on nerve function recovery after cervical spondylotic myelopathy surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 719-722. |
| [8] | Nie Huijuan, Huang Zhichun. The role of Hedgehog signaling pathway in transforming growth factor beta1-induced myofibroblast transdifferentiation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 754-760. |
| [9] | Li Wenjing, Li Haobo, Liu Congna, Cheng Dongmei, Chen Huizhen, Zhang Zhiyong. Comparison of different bioactive scaffolds in the treatment of regenerative pulp of young permanent teeth [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 499-503. |
| [10] | Li Chenjie, Lü Linwei, Song Yang, Liu Jingna, Zhang Chunqiu. Measurement and statistical analysis of trabecular morphological parameters of titanium alloy peri-prosthesis under preload [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 516-520. |
| [11] | Zhou Jihui, Yao Meng, Wang Yansong, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Influence of novel nanoscaffolds on biological behaviors of neural stem cells and the related gene expression [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 532-536. |
| [12] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [13] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [14] | Liu Liu, Zhou Qingzhu, Gong Zhuo, Liu Boyan, Yang Bin, Zhao Xian. Characteristics and manufacturing techniques of collagen/inorganic materials for constructing tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 607-613. |
| [15] | Ye Haimin, Ding Linghua, Kong Weihao, Huang Zutai, Xiong Long. Role and mechanism of hierarchical microchanneled bone scaffolds in promoting osteogenesis and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 621-625. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||