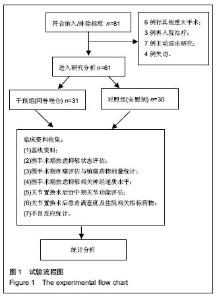
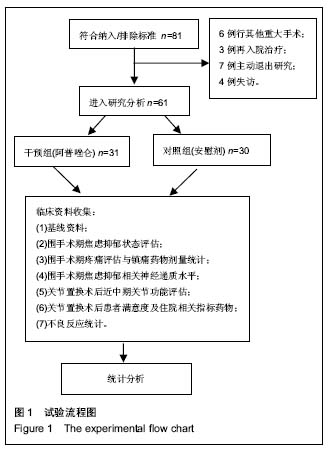
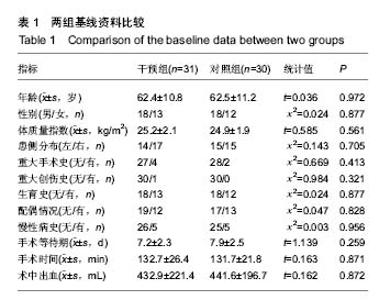
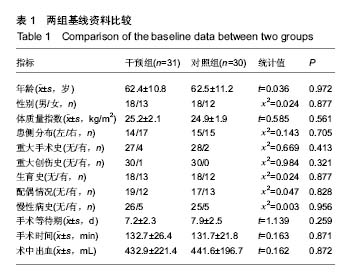
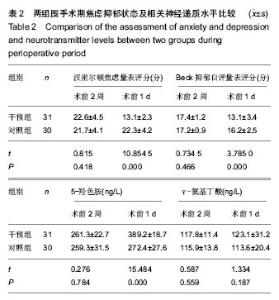
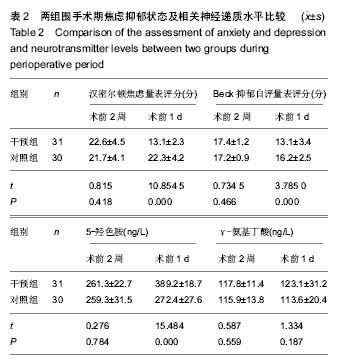
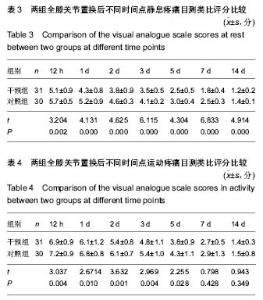
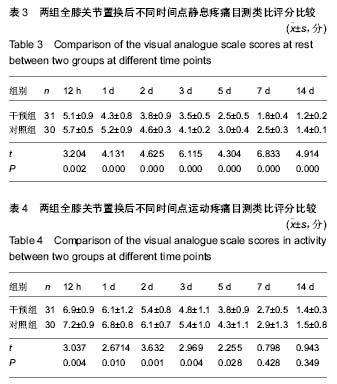
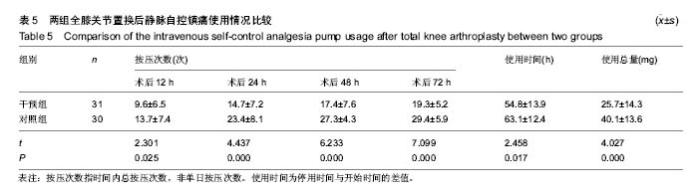
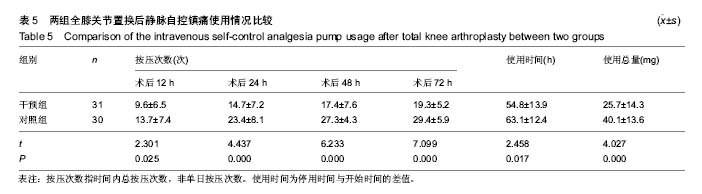
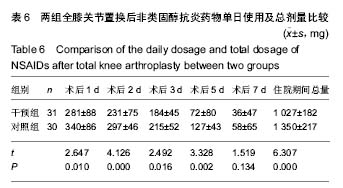
| [1] 马利平, 吴秋季, 谢东, 等.人工膝关节置换术后感染的诊断及治疗进展[J].中华医院感染学杂志, 2014,24(4): 1036-1038.[2] 黄自强, 孙长鲛. 膝关节置换患者手术失败原因的分析[J]. 中华医学杂志, 2015,95(20):1606-1608.[3] Poolman RW. ACP Journal Club. In knee OA, adding TKR to nonsurgical treatment improved pain and function at 12 mo. Ann Intern Med. 2016;164(2):C8.[4] Sakellariou VI, Poultsides LA, Ma Y, et al. Risk Assessment for Chronic Pain and Patient Satisfaction After Total Knee Arthroplasty. Orthopedics. 2016;39(1):55-62.[5] 沈彬, 翁习生, 廖刃, 等.中国髋、膝关节置换术加速康复--围术期疼痛与睡眠管理专家共识[J].中华骨与关节外科杂志, 2016, 9(2):91-97.[6] 王春生, 杨佩, 张子琦, 等. 疼痛灾难化对膝关节置换术后疗效的影响[J]. 中华关节外科杂志(电子版), 2014,8(6):698-701.[7] Lopez-de-Uralde-Villanueva I, Beltran-Alacreu H, Fernandez-Carnero J, et al. Widespread Pressure Pain Hyperalgesia in Chronic Nonspecific Neck Pain with Neuropathic Features: A Descriptive Cross-Sectional Study. Pain Physician. 2016;19(2):77-88.[8] Cunningham NR, Jagpal A, Tran ST, et al. Anxiety Adversely Impacts Response to Cognitive Behavioral Therapy in Children with Chronic Pain. J Pediatr. 2016; 171:227-233.[9] Curtin C. Pain Examination and Diagnosis. Hand Clin. 2016;32(1):21-26.[10] Kunz M, Hennig J, Karmann AJ, et al. Relationship of 5-HTTLPR Polymorphism with Various Factors of Pain Processing: Subjective Experience, Motor Responsiveness and Catastrophizing. PLoS One. 2016;11(4):e153089.[11] Du J, Zhang D, Yin Y, et al. The Personality and Psychological Stress Predict Major Adverse Cardiovascular Events in Patients With Coronary Heart Disease After Percutaneous Coronary Intervention for Five Years. Medicine (Baltimore). 2016;95(15):e3364.[12] O'Keefe-McCarthy S, Guo SL. Development of the Prodromal Symptoms-Screening Scale (PS-SS): Preliminary Validity and Reliability. Can J Cardiovasc Nurs. 2016;26(2):10-18.[13] Kim SH, Lee DH, Yoon KB, et al. Factors Associated with Increased Risk for Clinical Insomnia in Patients with Chronic Neck Pain. Pain Physician. 2015;18(6):593-598.[14] Millar L. Psychoactive Substance Dependence: A Dentist's Challenge. Prim Dent J. 2015;4(2):49-54.[15] 王坤正. 初次全膝关节置换术的临床现状[J]. 中国医师杂志, 2015,17(1):1-3.[16] Menendez ME, Baker DK, Oladeji LO, et al. Psychological Distress Is Associated with Greater Perceived Disability and Pain in Patients Presenting to a Shoulder Clinic. J Bone Joint Surg Am. 2015;97(24):1999-2003.[17] Dusad A, Pedro S, Mikuls TR, et al. Impact of Total Knee Arthroplasty as Assessed Using Patient-Reported Pain and Health-Related Quality of Life Indices: Rheumatoid Arthritis Versus Osteoarthritis. Arthritis Rheumatol. 2015;67(9): 2503-2511.[18] Martin C, Garbacz M, Bracard S. [Pain management in subarachnoid hemorrhages]. Soins. 2015;(801):19-21.[19] Dupuis LL, Lu X, Mitchell HR, et al. Anxiety, pain, and nausea during the treatment of standard-risk childhood acute lymphoblastic leukemia: A prospective, longitudinal study from the Children's Oncology Group. Cancer. 2016;122(7): 1116-1125.[20] Matsumoto S, Matsumoto K, Iida H. Transdermal fentanyl patch improves post-operative pain relief and promotes early functional recovery in patients undergoing primary total knee arthroplasty: a prospective, randomised, controlled trial. Arch Orthop Trauma Surg. 2015;135(9):1291-1297.[21] Liu GP, Xue FS, Sun C, et al. Electroacupuncture for pain treatment after total knee arthroplasty. Acupunct Med. 2015; 33(5):433.[22] van de Groes S, van der Ven P, Kremers-van DH, et al. Flexion and anterior knee pain after high flexion posterior stabilized or cruciate retaining knee replacement. Acta Orthop Belg. 2015;81(4):730-737. |