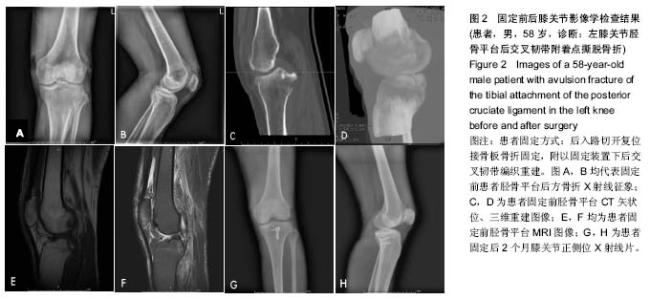
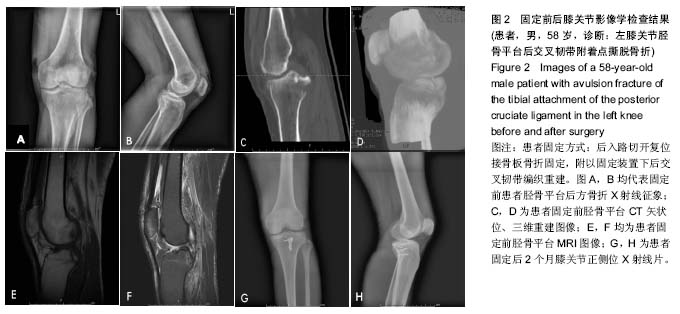
| [1] Zhang B, Cheng CK, Qu TB, et al. Partial versus intact posterior cruciate ligament-retaining total knee arthroplasty: a comparative study of early clinical outcomes. Orthop Surg. 2016;8(3):331-337. [2] Xu H, Chen YM, Zhai LF, et al. Surgical treatment of multiple ligament injuries of knee joints. Zhongguo Gu Shang. 2016; 29(5):456-459. [3] Lai Z, Liu ZX, Yang JL, et al. Clinical effect of staged repair and reconstruction of multiple ligament injuries in knee joints. Zhongguo Gu Shang. 2016;29(5):404-407. [4] Onishi Y, Hino K, Watanabe S, et al. The influence of tibial resection on the PCL in PCL-retaining total knee arthroplasty: a clinical and cadaveric study. J Orthop Sci. 2016;21(6): 798-803. [5] Chen SY, Chen CY, Chang SS, et al. Arthroscopic suture fixation fou avulsion fractures in the tibialattachment of the posterior cruciate ligament. Arthroscopy. 2012;28(10): 1454-1463.[6] 王健全,敖英芳,于长隆,等.经关节镜缝合治疗后交叉韧带胫骨止点撕脱骨折[J].郑州大学学报,2008,43(2):369-372.[7] Frosch KH, Balcarek P, Walde T, et al. A new posterolateral approach without fibula osteotomy for the treatment of tibial plateau fractures. J Orthop Trauma. 2010;24(8):515-520.[8] Nicandri GT, Klineberg EO, Wahl CJ, et al. Treatment of posterior cruciate ligament tibial avulsion fractures through a modified open posterior approach: operative technique and 12-to 48-month outcomes. J Orthop Trauma. 2008;22(5): 317-324.[9] Valis P, Repko M, Krbec M, et al. Surgical management of posterior cruciate ligament avulsion fracture. Acta Chir Orthop Traumatol Cech. 2008;75(1):34-39. [10] Yoo JH, Yang BK, Ryu HK. Lateral epicondylar femoral avulsion fracture combined with tibial fracture: a counterpart to the arcuate sign. Knee. 2008;15(1):71-74.[11] Kladny B, Albrecht C, Haase I, et al. Outcome of inpatient rehabilitation following total knee replacement using the HSS-Score. Z Orthop Ihre Grenzgeb. 2002;140(1):37-41.[12] Bengtsson J, Möllborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31.[13] Yin PB, Long AH, Shen J, et al. Treatment of intertrochanteric femoral fracture with proximal femo¬ral medial sustainable intramedullary nails: study protocol for a randomized controlled trial. Clin Transl Orthop. 2016; 1(2):44-50.[14] Wu T, Zhang GQ. Minimally invasive treatment of proximal humerus fractures with locking com¬pression plate improves shoulder function in older patients: study protocol for a prospective randomized controlled trial. Clin Transl Orthop. 2016;1(2):51-57.[15] Li Z. Minimally invasive closed reduction and internal fixation with fully threaded headless cannulated compression screws for repair of distal radius fracture: study protocol for a randomized controlled trial. Clin Transl Orthop. 2016;1(2): 58-63. |