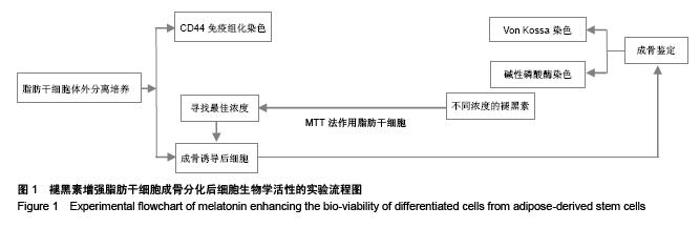
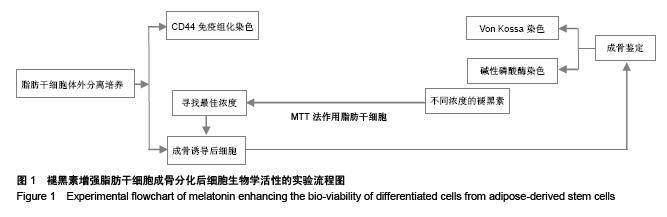
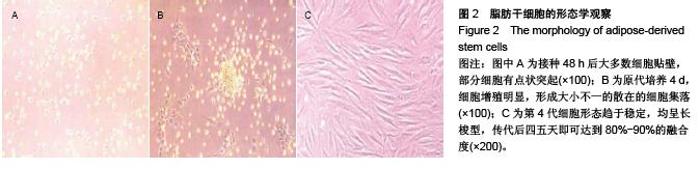
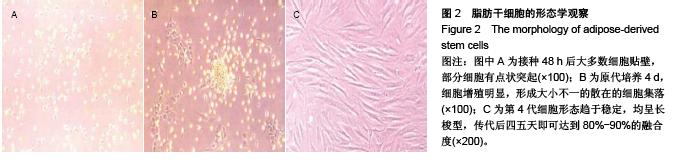
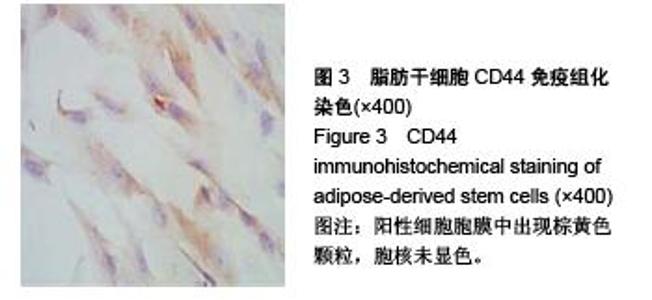
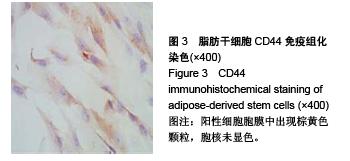
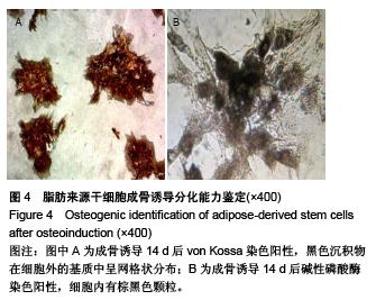
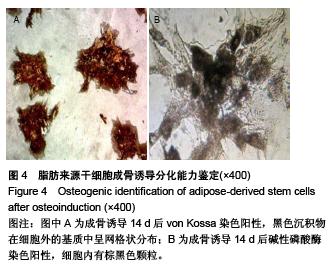
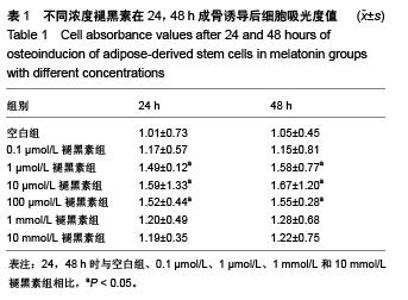
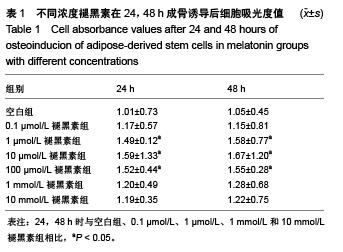
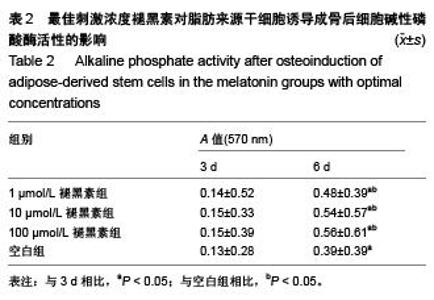
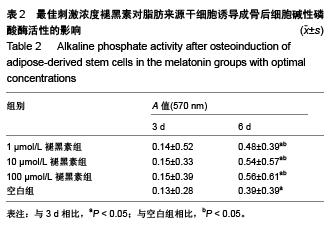
Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (50): 8072-8076.doi: 10.3969/j.issn.2095-4344.2015.50.007
Previous Articles Next Articles
Osteogenic differentiation of adipose-derived stem cells and the effect of melatonin on the bio-viability of differentiated cells
Lu Tan1, Wei Na2, Zhang Chao1, Dong Yu-zhen1
- 1Department of Orthopeadic Surgery, the First affiliated Hospital of Xinxiang Medical University, Weihui 453100, Henan Province, China; 2Second Department of Neurology, the Third Affiliated Hospital of Xinxiang Medical University, Xinxiang 453003, Henan Province, China
-
Received:2015-10-16Online:2015-12-03Published:2015-12-03 -
About author:Lu Tan, Studying for doctorate, Attending physician, Department of Orthopeadic Surgery, the First affiliated Hospital of Xinxiang Medical University, Weihui 453100, Henan Province, China -
Supported by:Stem Cells; Adipose Tissue; Cell Differentiation; Osteoblasts; Melatonin; Tissue Engineering
Funding: the Scientific Development Plan of Xinxiang City in 2015, No. 15SF27
CLC Number:
Cite this article
Lu Tan, Wei Na, Zhang Chao, Dong Yu-zhen. Osteogenic differentiation of adipose-derived stem cells and the effect of melatonin on the bio-viability of differentiated cells[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(50): 8072-8076.
share this article
| [1] Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211-228.[2] 梁笃,樊粤光,王海彬,等.成人脂肪来源干细胞定向诱导分化为软骨细胞的实验研究[J].广州中医药大学学报,2009,26(4): 412-418.[3] 徐丽丽,孙晓娟,郝秀仙,等. 在复合成分培养基中骨髓间充质干细胞的诱导成骨[J].中国组织工程研究,2015,19(10):1501- 1505.[4] 李龙,赵建辉,刘斌,等.兔脂肪干细胞的分离培养鉴定及诱导分化研究[J].口腔医学研究,2012,28(5):426-429.[5] 刘琴,王丽平,喻晶,等.组织块贴壁法扩增兔脂肪干细胞[J].中国组织工程研究,2014,18(1):88-93.[6] Cuzzocrea S, Reiter RJ. Pharmacological actions of melatonin in acute and chronic inflammation. Curr Top Med Chem. 2002;2(2):153-165.[7] Azeddine B, Letellier K, Wang da S, et al. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res. 2007;462:45-52.[8] Sánchez-Barceló EJ, Mediavilla MD, Tan DX, et al. Scientific basis for the potential use of melatonin in bone diseases: osteoporosis and adolescent idiopathic scoliosis. J Osteoporos. 2010;2010:830231.[9] Amstrup AK, Sikjaer T, Mosekilde L,et al. Melatonin and the skeleton. Osteoporos Int. 2013;24(12):2919-2927.[10] Man GC, Wang WW, Yeung BH, et al. Abnormal proliferation and differentiation of osteoblasts from girls with adolescent idiopathic scoliosis to melatonin. J Pineal Res. 2010; 49(1): 69-77.[11] Solá-Ruiz MF, Pérez-Martínez C, Martín-del-Llano JJ, et al. In vitro preliminary study of osteoblast response to surface roughness of titanium discs and topical application of melatonin. Med Oral Patol Oral Cir Bucal. 2015;20(1):e88-93.[12] Xiong XC, Zhu Y, Ge R, et al. Effect of Melatonin on the Extracellular-Regulated Kinase Signal Pathway Activation and Human Osteoblastic Cell Line hFOB 1.19 Proliferation.Int J Mol Sci. 2015;16(5):10337-10353.[13] Sun X, Shao Y, Jin Y, et al. Melatonin reduces bacterial translocation by preventing damage to the intestinal mucosa in an experimental severe acute pancreatitis rat model. Exp Ther Med. 2013;6(6):1343-1349.[14] 付洁,宋海岩,马传飞,等.褪黑素促进体外缺氧的神经干细胞增殖及定向分化[J].神经解剖学杂志,2015,31(1):37-43.[15] 丁华,袁志诚,耿荣科,等.骨髓间充质干细胞移植联用褪黑素对大鼠脊髓损伤修复的影响[J].郧阳医学院学报,2010,29(1):4-8.[16] Zaminy A, Ragerdi Kashani I, Barbarestani M, et al. Osteogenic differentiation of rat mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells: melatonin as a differentiation factor. Iran Biomed J. 2008;12(3):133-141.[17] Sethi S, Radio NM, Kotlarczyk MP, et al. Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J Pineal Res. 2010;49(3):222-238.[18] 何银锋,张鹏,赵国阳,等.铁过载对成骨细胞铁稳态及生物活性的影响[J].中华骨质疏松和骨矿盐疾病杂志,2013,6(1):37-43.[19] Zaminy A, Kashani IR, Barbarestani M, et al. Effects of melatonin on the proliferation and differentiation of rat adipose-derived stem cells.Indian J Plast Surg. 2008;41(1): 8-14. |
| [1] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| [2] | Chen Yang, Huang Denggao, Gao Yuanhui, Wang Shunlan, Cao Hui, Zheng Linlin, He Haowei, Luo Siqin, Xiao Jingchuan, Zhang Yingai, Zhang Shufang. Low-intensity pulsed ultrasound promotes the proliferation and adhesion of human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3949-3955. |
| [3] | Zhang Lishu, Liu Anqi, He Xiaoning, Jin Yan, Li Bei, Jin Fang. Alpl gene affects the therapeutic effect of bone marrow mesenchymal stem cells on ulcerative colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3970-3975. |
| [4] | Ruan Guangping, Yao Xiang, Liu-Gao Miyang, Cai Xuemin, Li Zian, Pang Rongqing, Wang Jinxiang, Pan Xinghua. Umbilical cord mesenchymal stem cell transplantation for traumatic systemic inflammatory response syndrome in tree shrews [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3994-4000. |
| [5] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [6] | Chen Lei, Zheng Rui, Jie Yongsheng, Qi Hui, Sun Lei, Shu Xiong. In vitro evaluation of adipose-derived stromal vascular fraction combined with osteochondral integrated scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3487-3492. |
| [7] | Wei Qin, Zhang Xue, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Jia Qiyu, Ma Chuang. Platelet-derived growth factor-BB induces the differentiation of rat bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2953-2957. |
| [8] | Chen Xiao, Guo Zhi, Chen Lina, Liu Xuanyong, Zhang Yihuizhi, Li Xumian, Wang Yueqiao, Wei Liya, Xie Jing, Lin Li. Factors affecting the mobilization and collection of autologous peripheral blood hematopoietic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2958-2962. |
| [9] | Guo Zhibin, Wu Chunfang, Liu Zihong, Zhang Yuying, Chi Bojing, Wang Bao, Ma Chao, Zhang Guobin, Tian Faming. Simvastatin stimulates osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2963-2968. |
| [10] | Li Congcong, Yao Nan, Huang Dane, Song Min, Peng Sha, Li Anan, Lu Chao, Liu Wengang. Identification and chondrogenic differentiation of human infrapatellar fat pad derived stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2976-2981. |
| [11] | Gao Yuanhui, Xiang Yang, Cao Hui, Wang Shunlan, Zheng Linlin, He Haowei, Zhang Yingai, Zhang Shufang, Huang Denggao. Comparison of biological characteristics of adipose derived mesenchymal stem cells in Wuzhishan inbreed miniature pigs aged two different months [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2988-2993. |
| [12] | Cao Yang, Zhang Junping, Peng Li, Ding Yi, Li Guanghui. Isolation and culture of rabbit aortic endothelial cells and biological characteristics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3000-3003. |
| [13] | Dai Min, Wang Shuai, Zhang Nini, Huang Guilin, Yu Limei, Hu Xiaohua, Yi Jie, Yao Li, Zhang Ligang. Biological characteristics of hypoxic preconditioned human amniotic mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3004-3008. |
| [14] | Qin Yanchun, Rong Zhen, Jiang Ruiyuan, Fu Bin, Hong Xiaohua, Mo Chunmei. Chinese medicine compound preparation inhibits proliferation of CD133+ liver cancer stem cells and the expression of stemness transcription factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3016-3023. |
| [15] | Dai Yaling, Chen Lewen, He Xiaojun, Lin Huawei, Jia Weiwei, Chen Lidian, Tao Jing, Liu Weilin. Construction of miR-146b overexpression lentiviral vector and the effect on the proliferation of hippocampal neural stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3024-3030. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||