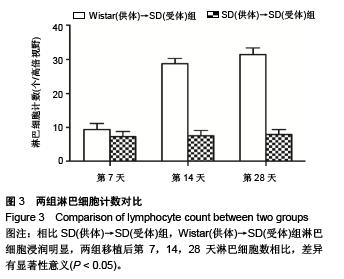
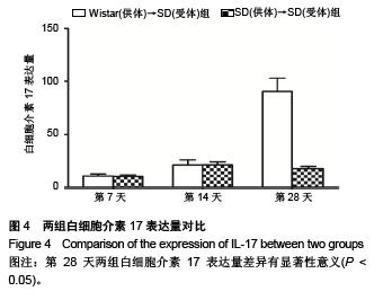
Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (49): 8010-8014.doi: 10.3969/j.issn.2095-4344.2015.49.024
Previous Articles Next Articles
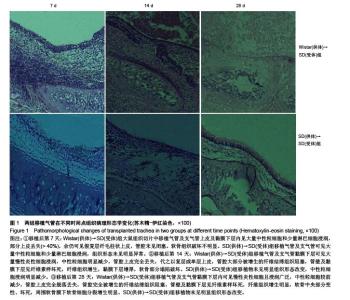
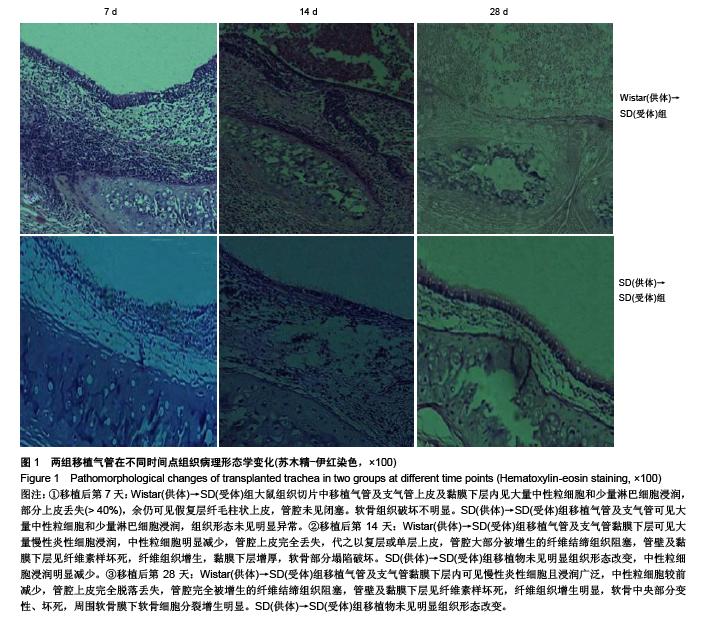
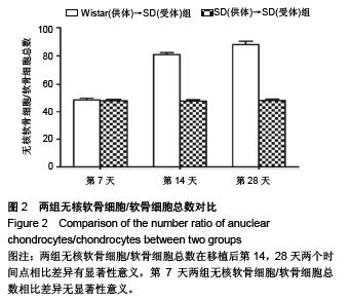
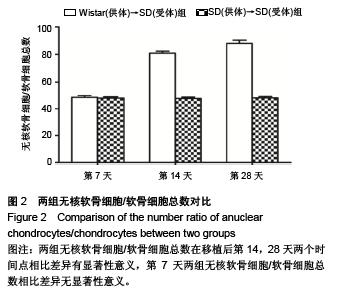
Establishment of rat models of chronic rejection after heterotopic tracheal transplantation and the relationship with interleukin-17
Wu Dong1, Li Xiao-jun2, Liu Mao-lin3, Wang Jian-jun1, Zhai Wei1
- 1Department of Cardiothoracic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China; 2Department of Cardiothoracic Surgery, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China; 3Dongfeng General Hospital of Shiyan City, Shiyan 442000, Hubei Province, China
-
Received:2015-09-07Online:2015-11-30Published:2015-11-30 -
Contact:Zhai Wei, M.D., Associate professor, Department of Cardiothoracic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China -
About author:Wu Dong, Studying for master’s degree, Department of Cardiothoracic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China Li Xiao-jun, Master, Physician, Department of Cardiothoracic Surgery, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong Province, China Wu Dong and Li Xiao-jun contributed equally to this article -
Supported by:the National Natural Science Foundation of China, No. 30700767; the Foundation of the Ministry of Education of China for Returned Overseas Chinese Scholars, No. 2008890; the Natural Science Foundation of Hubei Province of China, No. 2013CFB088
CLC Number:
Cite this article
Wu Dong, Li Xiao-jun, Liu Mao-lin, Wang Jian-jun, Zhai Wei. Establishment of rat models of chronic rejection after heterotopic tracheal transplantation and the relationship with interleukin-17[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(49): 8010-8014.
share this article
| [1] Hertz MI, Aurora P, Christie JD, et al. Scientific Registry of the International Society for Heart and Lung Transplantation: introduction to the 2009 Annual Reports. J Heart Lung Transplant. 2009;28(10):989-992. [2] Sundaresan S, Trulock EP, Mohanakumar T, et al. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. Washington University Lung Transplant Group.Ann Thorac Surg. 1995;60(5):1341-1346. [3] Marck KW, Prop J, Wildevuur CR, et al. Lung transplantation in the rat: histopathology of left lung iso- and allografts. J Heart Transplant. 1985;4(2):263-266. [4] Sato M, Keshavjee S, Liu M. Translational research: animal models of obliterative bronchiolitis after lung transplantation. Am J Transplant. 2009;9(9):1981-1987. [5] Boehler A, Chamberlain D, Kesten S, et al. Lymphocytic airway infiltration as a precursor to fibrous obliteration in a rat model of bronchiolitis obliterans. Transplantation. 1997;64(2): 311-317. [6] Hele DJ, Yacoub MH, Belvisi MG. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis Respir Res. 2001;2(3):169-183. [7] Hausen B, Demertzis S, Schröder F, et al. Double-lung transplantation in the rat: an acute, syngeneic in situ model. Ann Thorac Surg. 1996;61(1):184-189. [8] Reinsmoen NL, Bolman RM, Savik K, et al. Are multiple immunopathogenetic events occurring during the development of obliterative bronchiolitis and acute rejection. Transplantation. 1993;55(5):1040-1044. [9] 张临友,王俊峰,张学忠,等.大鼠气管移植后肺移植慢性排斥反应模型的建立[J].中华实验外科杂志, 2004, 21(12):1561. [10] Maasilta P, Salminen US, Taskinen E, et al. Obliterative airway disease in a porcine heterotopic bronchial allograft model. Transpl Int. 2000;13(3):218-224. [11] Beigi FS, West LJ. Site of heterotopic tracheal allograft Implantation affects graft rejection pattern. J Heart Lung Transplant. 2003; 22(1): 104. [12] Ryu S, Lee JH, Kim SI. IL-17 increased the production of vascular endothelial growth factor in rheumatoid arthritis synoviocytes. Clin Rheumatol. 2006;25(1):16-20. [13] Sergejeva S, Ivanov S, Lötvall J, et al. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005; 33(3):248-253. [14] Rangel-Moreno J, Carragher DM, de la Luz Garcia- Hernandez M, et al. The development of inducible bronchus- associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639-646. [15] Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation Am J Transplant. 2011;11(5):911-922. |
| [1] | Chen Ziyang, Pu Rui, Deng Shuang, Yuan Lingyan. Regulatory effect of exosomes on exercise-mediated insulin resistance diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4089-4094. |
| [2] | Chen Yang, Huang Denggao, Gao Yuanhui, Wang Shunlan, Cao Hui, Zheng Linlin, He Haowei, Luo Siqin, Xiao Jingchuan, Zhang Yingai, Zhang Shufang. Low-intensity pulsed ultrasound promotes the proliferation and adhesion of human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3949-3955. |
| [3] | Yang Junhui, Luo Jinli, Yuan Xiaoping. Effects of human growth hormone on proliferation and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3956-3961. |
| [4] | Sun Jianwei, Yang Xinming, Zhang Ying. Effect of montelukast combined with bone marrow mesenchymal stem cell transplantation on spinal cord injury in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3962-3969. |
| [5] | Gao Shan, Huang Dongjing, Hong Haiman, Jia Jingqiao, Meng Fei. Comparison on the curative effect of human placenta-derived mesenchymal stem cells and induced islet-like cells in gestational diabetes mellitus rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3981-3987. |
| [6] | Hao Xiaona, Zhang Yingjie, Li Yuyun, Xu Tao. Bone marrow mesenchymal stem cells overexpressing prolyl oligopeptidase on the repair of liver fibrosis in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3988-3993. |
| [7] | Liu Jianyou, Jia Zhongwei, Niu Jiawei, Cao Xinjie, Zhang Dong, Wei Jie. A new method for measuring the anteversion angle of the femoral neck by constructing the three-dimensional digital model of the femur [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3779-3783. |
| [8] | Meng Lingjie, Qian Hui, Sheng Xiaolei, Lu Jianfeng, Huang Jianping, Qi Liangang, Liu Zongbao. Application of three-dimensional printing technology combined with bone cement in minimally invasive treatment of the collapsed Sanders III type of calcaneal fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3784-3789. |
| [9] | Qian Xuankun, Huang Hefei, Wu Chengcong, Liu Keting, Ou Hua, Zhang Jinpeng, Ren Jing, Wan Jianshan. Computer-assisted navigation combined with minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3790-3795. |
| [10] | Hu Jing, Xiang Yang, Ye Chuan, Han Ziji. Three-dimensional printing assisted screw placement and freehand pedicle screw fixation in the treatment of thoracolumbar fractures: 1-year follow-up [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3804-3809. |
| [11] | Shu Qihang, Liao Yijia, Xue Jingbo, Yan Yiguo, Wang Cheng. Three-dimensional finite element analysis of a new three-dimensional printed porous fusion cage for cervical vertebra [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3810-3815. |
| [12] | Wang Yihan, Li Yang, Zhang Ling, Zhang Rui, Xu Ruida, Han Xiaofeng, Cheng Guangqi, Wang Weil. Application of three-dimensional visualization technology for digital orthopedics in the reduction and fixation of intertrochanteric fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3816-3820. |
| [13] | Sun Maji, Wang Qiuan, Zhang Xingchen, Guo Chong, Yuan Feng, Guo Kaijin. Development and biomechanical analysis of a new anterior cervical pedicle screw fixation system [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3821-3825. |
| [14] | Lin Wang, Wang Yingying, Guo Weizhong, Yuan Cuihua, Xu Shenggui, Zhang Shenshen, Lin Chengshou. Adopting expanded lateral approach to enhance the mechanical stability and knee function for treating posterolateral column fracture of tibial plateau [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3826-3827. |
| [15] | Zhu Yun, Chen Yu, Qiu Hao, Liu Dun, Jin Guorong, Chen Shimou, Weng Zheng. Finite element analysis for treatment of osteoporotic femoral fracture with far cortical locking screw [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3832-3837. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||