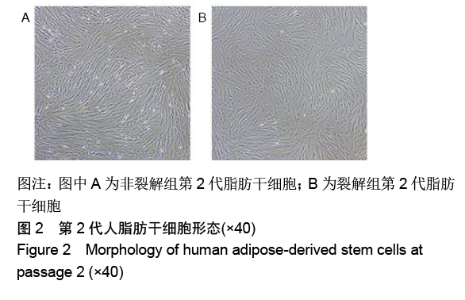
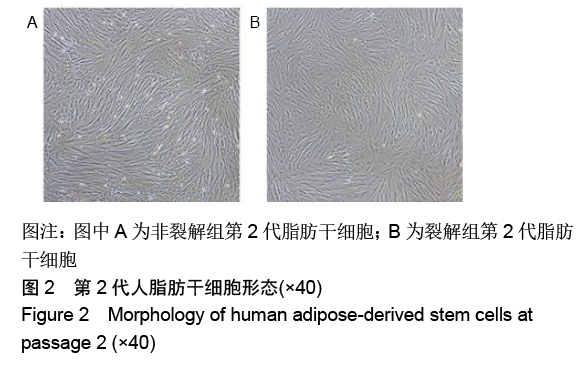
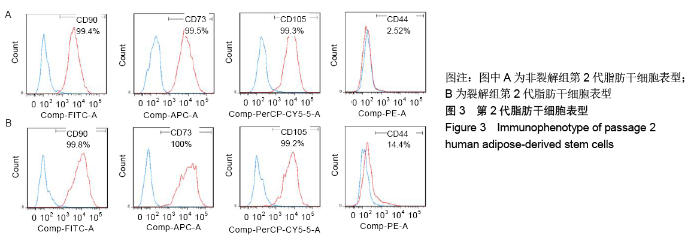
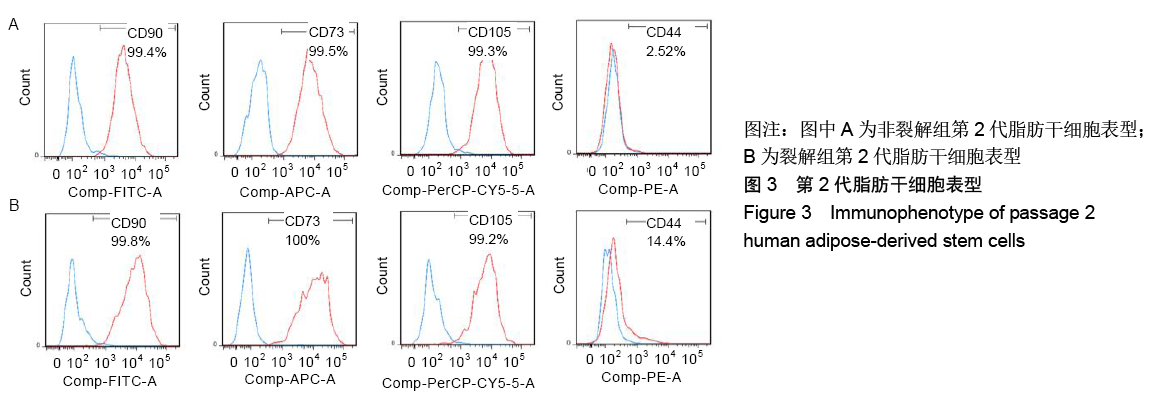
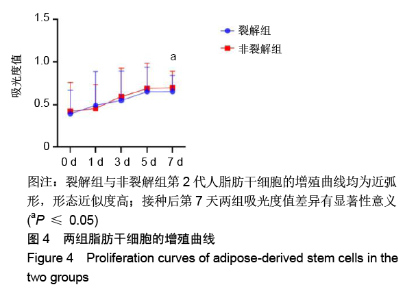
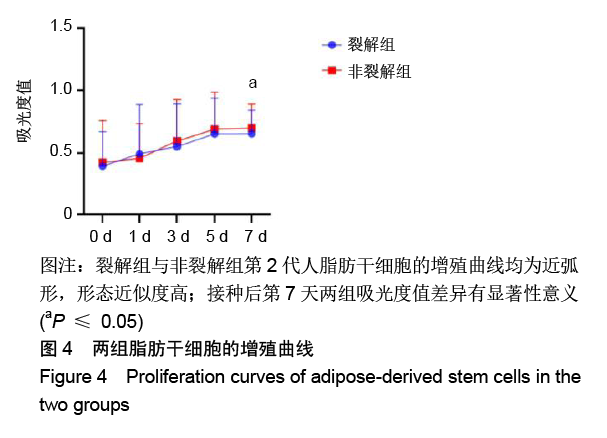
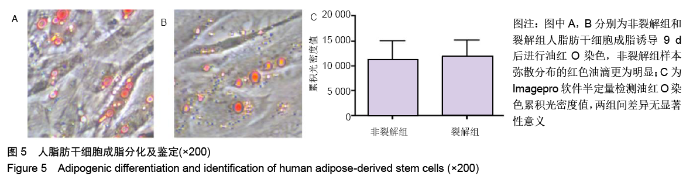
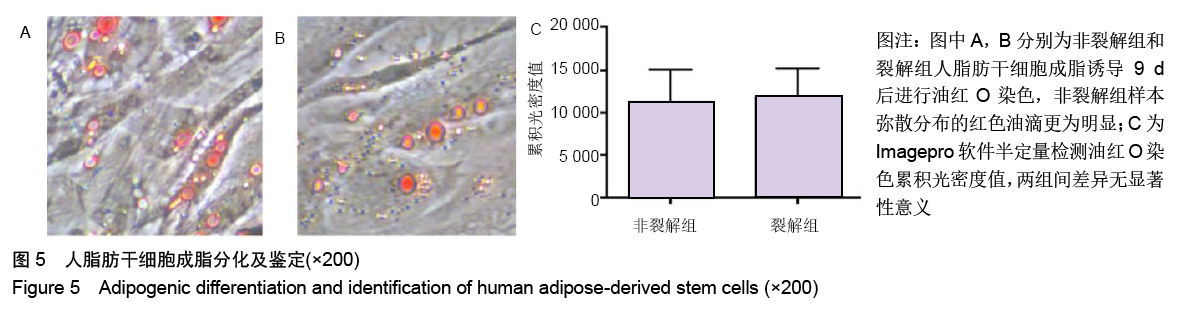
[1] KIM BS, OTT V, BOECKER AH, et al. The Effect of Antiseptics on Adipose-Derived Stem Cells. Plast Reconstr Surg. 2017;139(3): 625-637.
[2] GIMBLE JM, KATZ AJ, BUNNELL BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249-1260.
[3] NADERI N, COMBELLACK EJ, GRIFFIN M, et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. 2017;14(1):112-124.
[4] RIIS S, ZACHAR V, BOUCHER S, et al. Critical steps in the isolation and expansion of adipose-derived stem cells for translational therapy. Expert Rev Mol Med. 2015;17:e11.
[5] ARRIZABALAGA JH, NOLLERT MU. Properties of porcine adipose- derived stem cells and their applications in preclinical models. Adipocyte. 2017;6(3):217-223.
[6] GRASYS J, KIM BS, PALLUA N. Content of Soluble Factors and Characteristics of Stromal Vascular Fraction Cells in Lipoaspirates from Different Subcutaneous Adipose Tissue Depots. Aesthet Surg J. 2016; 36(7):831-841.
[7] KERN S, EICHLER H, STOEVE J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294-1301.
[8] HU X, FU Y, ZHANG X, et al. Histone deacetylase inhibitor sodium butyrate promotes the osteogenic differentiation of rat adipose-derived stem cells. Dev Growth Differ. 2014;56(3):206-213.
[9] HOLM JS, TOYSERKANI NM, SORENSEN JA. Adipose-derived stem cells for treatment of chronic ulcers: current status. Stem Cell Res Ther. 2018;9(1):142.
[10] KOMIYAMA S, SAKAKURA C, MURAYAMA Y, et al. Adipose-derived stem cells enhance tissue regeneration of gastrotomy closure. J Surg Res. 2013;185(2):945-952.
[11] LIAO N, ZHENG Y, XIE H, et al. Adipose tissue-derived stem cells ameliorate hyperglycemia, insulin resistance and liver fibrosis in the type 2 diabetic rats. Stem Cell Res Ther. 2017;8(1):286.
[12] MA T, SUN J, ZHAO Z, et al. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res Ther. 2017;8(1):124.
[13] LI Y, ZHANG W, GAO J, et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7(1):102.
[14] GIANCOLA R, BONFINI T, IACONE A. Cell therapy: cGMP facilities and manufacturing. Muscles Ligaments Tendons J. 2012;2(3):243-247.
[15] HORN P, BORK S, DIEHLMANN A, et al. Isolation of human mesenchymal stromal cells is more efficient by red blood cell lysis. Cytotherapy. 2008;10(7):676-685.
[16] WILSON A, SHEHADEH LA, YU H, et al. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics. 2010;11:229.
[17] JUSTILIEN V, REGALA RP, TSENG IC, et al. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS One. 2012;7(4):e35040.
[18] GHANEKAR A, AHMED S, CHEN K, et al. Endothelial cells do not arise from tumor-initiating cells in human hepatocellular carcinoma. BMC Cancer. 2013;13:485.
[19] SHAWCROSS DL, SHABBIR SS, TAYLOr NJ, et al. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51(3):1062-1069.
[20] WANG Q, WANG Y, YU Z, et al. Ammonia-induced energy disorders interfere with bilirubin metabolism in hepatocytes. Arch Biochem Biophys. 2014;555-556:16-22.
[21] LI SH, LIAO X, ZHOU TE, et al. Evaluation of 2 Purification Methods for Isolation of Human Adipose-Derived Stem Cells Based on Red Blood Cell Lysis With Ammonium Chloride and Hypotonic Sodium Chloride Solution. Ann Plast Surg. 2017;78(1):83-90.
[22] HASSELL T, GLEAVE S, BUTLER M. Growth inhibition in animal cell culture. The effect of lactate and ammonia. Appl Biochem Biotechnol. 1991;30(1):29-41.
[23] PAREKKADAN B, SETHU P, VAN POLL D, et al. Osmotic selection of human mesenchymal stem/progenitor cells from umbilical cord blood. Tissue Eng. 2007;13(10):2465-2473. |