Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (31): 5056-5061.doi: 10.3969/j.issn.2095-4344.0378
Previous Articles Next Articles
The latest advances in surgical treatment of osteonecrosis of femoral head: how to achieve the goal of hip joint preserving treatment
Wang Jun, Ge Qiao-feng, Wu Zhuang-zhuang, Lü Zhi
- Department of Orthopedics, Second Clinical Medical School of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Online:2018-11-08Published:2018-11-08 -
Contact:Lü Zhi, MD, Chief physician, Professor, Department of Orthopedics, Second Clinical Medical School of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Wang Jun, Master candidate, Department of Orthopedics, Second Clinical Medical School of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:the Natural Science Foundation of Shanxi Province, No. 2015011097
CLC Number:
Cite this article
Wang Jun, Ge Qiao-feng, Wu Zhuang-zhuang, Lü Zhi . The latest advances in surgical treatment of osteonecrosis of femoral head: how to achieve the goal of hip joint preserving treatment [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(31): 5056-5061.
share this article

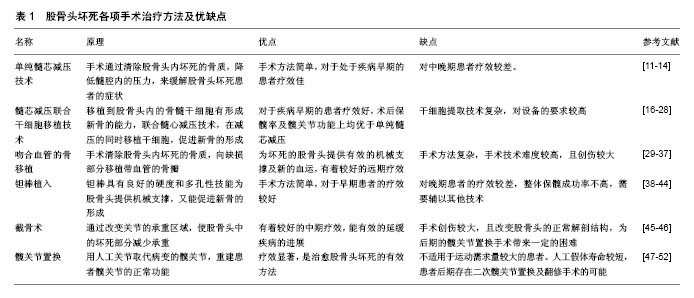
2.1 单纯髓芯减压技术 髓芯减压技术的具体病理生理机制目前尚不清楚,但有研究表明髓芯减压技术通过清除坏死的骨质,缓解股骨头内部的高压力,并且刺激隧道内骨细胞的生长[11]。现阶段来看,髓芯减压是治疗股骨头坏死应用最为广泛的手术方式[12]。对于此治疗方法的具体疗效大量学者进行了研究。Marker等[13]对接受单纯髓芯减压治疗的研究进行了系统回顾,研究中共纳入1 268例患髋,结果显示术后70%的髋关节无需进一步手术处理,且63%髋关节在术后的随访中未发生恶化,因此认为单纯髓芯减压的治疗是有效的。为了明确髓芯减压治疗的具体适应证,Rajagopal等[14]回顾分析了139例接受髓芯减压治疗的患者,结果发现在关节坏死面积小于50%的患者中疼痛症状缓解较明显。Zhao等[15]研究并分析了67例病变处于早期的原发性股骨头坏死患者,均接受单纯髓芯减压治疗,术后平均随访5年,结果发现大部分患者的髋关节功能明显改善且达到了保髋的目的。研究中有6例髋关节保髋失败,分析后发现这些患髋均存在血供障碍。研究者认为,髓芯减压技术对病变处于早期的髋关节疗效较好,但是股骨头的血供是否存在障碍直接影响治疗的效果。 2.2 髓芯减压联合干细胞移植技术 单纯髓芯减压技术短期疗效尚可,但中远期疗效常不能令人满意[16]。由于髋关节不恰当的骨重构,术后保髋成功率较低,研究者尝试髓芯减压联合其他治疗方式以改善其远期疗效[17]。 干细胞具有增殖分化成其他细胞的能力,在不同的生长环境下干细胞分化成特定的组织细胞,修复组织缺损[18]。研究者应用干细胞移植技术治疗股骨头坏死,促进坏死区域新骨形成[19-20]。Chen等[21]研究9例病变处于早期的髋关节,研究中患者均接受动脉注射骨髓间充质干细胞移植,术后随访24个月,结果发现患者股骨头的坏死面积均较术前明显减少,髋关节疼痛评分较术前明显减低。Mao等[22]对62例患者(78髋)进行了经旋股内侧动脉注射自体骨髓干细胞的治疗,术后随访5年,发现92.31%的髋关节目测类比疼痛评分降低、髋关节功能评分增高,认为干细胞移植技术在股骨头坏死的治疗中具有较高的安全性和疗效。 移植到股骨头内的骨髓干细胞具有形成新骨的能力,尝试通过髓芯减压通道在减压的同时向股骨头内移植骨髓干细胞,促进坏死区域新骨的形成[23]。Tabatabaee等[24]对坏死病变处于早期的28例髋关节进行研究,将患者随机分为骨髓干细胞移植与髓芯减压技术相结合的治疗组和单纯髓芯减压的对照组。术后随访2年,结果发现,虽然两组患者的WOMAC骨关节炎指数评分及疼痛评分均有改善,但在影像学的表现上,联合治疗组较单纯减压组的改善情况更为显著。认为髓芯减压联合干细胞移植对早期股骨头坏死的治疗是有效的。干细胞移植技术不仅可以改善单纯髓芯减压治疗的疗效,对于其他治疗方法如髓芯减压联合骨移植,也有一定的促进作用。Ma等[25]对53例股骨头坏死的髋关节进行了研究,患者随机分为髓芯减压联合自体骨加骨髓间充质干细胞移植组(治疗组)和髓芯减压联合自体骨移植组(对照组)。术后随访2年,结果发现虽然2组患者的髋关节功能及疼痛方面均较术前有显著改善,但是在疾病进展方面,对照组中的疾病进展率高于治疗组。对于病变处于早期的髋关节,对照组仅有66.7%的髋关节未发生进展,而治疗组全未发生进展。该作者认为骨髓间充质干细胞移植技术在延缓疾病进展方面有积极作用。 但是,移植后的干细胞分化率较低,新骨的生成情况仍不能让人满意[26]。有研究者进行了辅助应用生长因子和提高移植干细胞浓度等方法来提高新骨生成率的相关探索。Nandeesh等[27]报告了48例髋关节早期病变的患者,均接受股骨头髓芯减压加骨髓干细胞联合生长因子移植的治疗方法,术后随访2年,结果发现MRI下93%的髋关节出现了新骨的形成。Rastogi等[28]选取了60例股骨头坏死处的髋关节,随机分为2组,分别接受髓芯减压联合体外分离扩增的骨髓间充质干细胞移植组(治疗组)和髓芯减压联合未经过分离处理的骨髓移植组(对照组)。术后随访2年,结果显示虽然2组的HHS髋关节评分均有显著提高,但是在影像学表现上,治疗组中髋关节坏死面积未见明显进展,在对照组中髋关节的坏死面积显示出了不同程度的的增大。研究者认为提高移植干细胞浓度的方法可以增加术后新骨的生成。 从目前的研究看,髓芯减压联合干细胞移植的治疗方法在远期预后及促进新骨生成的方面要优于单纯的髓芯减压治疗。 2.3 吻合血管的骨移植 髓芯减压后由于股骨头下缺乏有效的物理支撑,常造成关节面的塌陷[29]。研究者尝试对坏死的股骨头进行皮质骨移植,认为移植的皮质骨不仅可以对股骨头形成有效物理支撑,还可以一定程度上诱导新骨形成[30]。但是移植的骨质自身不具有血供,坏死的股骨头本身血运也不佳,皮质骨移植后的骨存活情况难以令人满意[31]。为了解决移植骨的血供问题,尝试移植带血管的自体骨,在支撑关节面的同时提供新的血运、促进新骨的形成[32]。手术方法为:清除股骨头下坏死的骨质,给予坏死区域充分的减压,选用带血管的腓骨或髂骨填补空缺,利用显微外科技术吻合移植骨瓣的血管[33]。 为了探讨骨瓣移植的具体疗效,研究者进行了大量的随机对照研究。Liqh等[34]对近年来腓骨瓣治疗股骨头坏死的研究进行了荟萃分析,共纳入了21项研究,2 709患者,3 486患髋,结果发现患者术后髋关节功能评分平均提高了21.7分,且约1/2的髋关节在术后影像学上显示有新骨形成,认为腓骨瓣移植是一种有效的治疗股骨头坏死的方法。Fontecha等[35]研究并分析了9例(10髋)股骨头坏死的青少年患者,研究中患者均接受吻合血管的腓骨移植治疗,术后至少随访2年,研究发现:治疗后患髋的HHS评分由原来的平均37.2分改善至平均92.3分。术后行SPECT/CT检查显示股骨头下均出现了或多或少的新骨形成。Cao等[36]研究并分析了27例股骨头坏死的患者(54髋),研究中患者随机分为髓芯减压治疗组和减压联合带血管的腓骨瓣移植治疗组,术后随访36个月,结果发现:在HHS关节评分、核磁共振的影像学表现上、SPECT/CT的影像学表现上,腓骨瓣移植组新骨的生成情况均优于单纯髓芯减压组。 另外,之前的研究随访时间多为1-3年,对于腓骨瓣移植后远期效果的评价研究较少。为反映该治疗方法的远期疗效,近年来有研究者进行了术后长时间的随访研究。Unal等[37]随访并研究了21例患者,研究中患者均接受游离的腓骨瓣移植治疗,随访7年以上,结果发现患者术后HHS的评分较术前有明显的提高,且早期患者的疗效要要优于晚期患者的疗效。该作者认为游离腓骨瓣移植对于股骨头坏死的治疗有较好的远期疗效,且疾病分期越早,治疗效果越好。 从目前的研究看,吻合血管的骨移植在诱导新骨形成以及改善疾病远期预后方面要优于单纯髓芯减压。但由于该术式对患者创伤较大,且此治疗方法对显微外科技术要求较高,临床上开展起来较困难,总体研究较少。 2.4 钽棒植入技术 钽棒技术主要是通过多孔钽棒的硬度对股骨头下提供有效的机械支撑,并且凭借钽棒的多孔性和显微结构上类似于松质骨的形态,在提供有效机械支撑的同时促进股骨头内新骨的形成[38]。 随着科技的发展,近年来有研究者用CT导航技术来辅助并指导术中置入钽棒,以此尝试缩短手术时间,减少手术创伤,并取得了一定的成果。Hu等[39]利用实时 CT导航技术指导钽棒植入来治疗股骨头坏死。研究共纳入24例(29髋)原发性股骨头坏死的患者。术后平均随访5.4年,结果发现使用这种方法可以减少术中出血量和手术时间,且术后患者的症状也得到了明显改善。该作者认为实时CT导航技术对钽棒植入手术有明显的收益,是一种安全有效的技术。 但是单纯的钽棒植入技术治疗结果并不理想,有学者进行了联合其他技术如干细胞移植、骨移植等治疗的研究[40]。Mao等[41]对59例股骨头坏死患者(89髋)进行了研究。研究中患者随机分为钽棒植入联合干细胞移植的治疗组和单纯钽棒植入的对照组,术后随访36个月,结果发现对照组中有9例髋关节最终需要行人工关节置换,在治疗组中仅有3例需要进行人工关节置换。且无论是在术后功能评分上还是影像学表现上,治疗组均优于对照组,研究者认为钽棒与干细胞移植相结合的治疗方法可能是一种安全且疗效显著的治疗方法。Liu等[42]研究分析了94例接受髓芯减压、钽棒植入联合骨移植治疗的病人,术后平均随访35.4个月,结果发现这种联合治疗的方法对于股骨头坏死处于早期的患者疗效较好。 此外,钽棒技术还可以与多种保髋治疗方法联合起来,用于治疗疗效较差的晚期患者,以期达到保髋的目的。 Zhao等[43]对24例(31髋)股骨头坏死处于晚期的患者进行了研究,患者接受的治疗方法均为钽棒植入联合骨髓间充质干细胞移植加吻合血管的髂骨移植。术后进行平均随访5.3年,结果发现,术后总体的保髋成功率达到80%。该作者认为这种联合治疗方法可以有效治疗股骨头坏死晚期患者。 就目前的研究结果看,钽棒技术对病变处于早期髋关节的治疗是安全有效的。但是对晚期髋关节的治疗效果不佳,需要辅以其他技术来提高保髋率。 2.5 截骨术 截骨术主要是通过截骨的方法来改变股骨头的力线,进而改变关节的承重区域,使股骨头中的坏死部分减少承重,延缓疾病发展至末期。目前对截骨术的研究较少,原因可能有[44]:①截骨术的创伤较大,常被患者拒绝;②截骨术会改变股骨转子间的正常解剖结构,给后期的关节置换带来一定困难,常不被术者所采纳。为了明确截骨术治疗的远期疗效,研究者进行了探讨与研究。Baba等[45]研究并分析了36例(46髋)年轻的股骨头坏死患者,均接受股骨转子间旋转截骨术,术后平均随访16.1年,结果发现术后患者的髋关节功能有明显改善,术后10,15,20年的保髋成功率依次为91.1%,75.5%,67.9%。该作者认为从长期的随访结果上来看,通过截骨来改变股骨头负重区的方法来治疗股骨头坏死是有效的。 但是,截骨术总的保髋率仍有待提高。为了提高保髋率,研究者对导致治疗失败的多种因素进行了研究。Sonoda等[46]分析了28例(28髋)创伤后股骨头坏死的患者,患者均接受转子间截骨治疗,术后平均随访12.3年,根据术后是否行髋关节置换将患者分为髋关节存活组和髋关节置换组,分别比较各个因素对两组的不同影响。结果发现大部分患者保髋成功。其中术前髋关节自身较晚的分期以及术后负重区完整关节面的比率小于33.6%是保髋成功与否的危险因素。Okura等[47]研究并分析了102例接受转子间旋转截骨术治疗的髋关节,术后平均随访10.1年,发现髋关节HHS评分由术前平均10.0分提高到术后平均88.1分,研究中有27髋出现了坏死面积的增大,11例髋关节保髋失败。在多因素模型下分析保髋失败的影响因素,结果显示术后负重区完整关节面的比率小于33.3%以及CE角小于25°是保髋失败的独立危险因素,该作者认为这两点因素应该在行截骨手术前被予以特殊考虑。 从目前的研究来看,截骨术相较于单纯髓芯减压治疗,有较好的长期疗效。影响其保髋成功率的具体因素仍不明确,有待于研究者进行进一步考究。 2.6 髋关节置换 当疾病发展至末期时,股骨头表面塌陷,髋关节功能严重丧失,人工关节置换是恢复关节功能的关键。由于人工关节面的磨损以及磨损后碎屑产生的溶骨反应,使得人工关节的使用寿命较短,常为10-20年[48]。为了克服人工关节使用寿命不长的问题,近年来研发出了以第3代氧化铝陶瓷为材料的人工关节。这种人工关节以其出色的抗磨损性以及非常低的组织排斥性,显著增加了人工关节的使用寿命,更适合于年轻和年老但有大运动量需求的患者[49]。 Kim等[50]研究了277例(334髋)年龄小于50岁的全髋关节置换患者,所有患者均使用第3代氧化铝陶瓷为材料的人工关节,术后平均随访13.1年。研究发现术后患者的平均HHS评分为93分,33例髋关节出现了“咔哒”的声音,2例出现了“吱吱”的磨损声音,但术后均未发现髋部骨质的溶解及陶瓷头或内衬的破裂。另有报道发现第3代氧化铝陶瓷人工假体明显改善术后髋关节功能评分,更重要的是,术后所有髋关节均未发现明显的骨质溶解,且均未行髋关节翻修手术[51]。Byun等[52]回顾性分析了41例(56髋)处于疾病晚期的年轻的股骨头坏死患者,研究中患者均进行全髋关节置换术,人工髋臼和股骨头的材料选择均为陶瓷,术后平均随访7.7年。结果发现术后髋关节的功能较术前均有很大改善,患髋的影像学表现上均未发现明显的骨质溶解及假体松脱。 氧化铝陶瓷假体的出现,使得全髋关节置换术的适应证逐渐放宽,目前发现的术后并发症主要以关节的异响声为主。但是对于氧化铝陶瓷关节的具体寿命以及并发症的主要原因,现阶段的研究较少,期待对该治疗方法进行更深一步的研究,以对其具体的适应证和禁忌证做出更好的定义。表1。"
| [1] Zalavras CG,Lieberman JR.Osteonecrosis of the femoral head: evaluation and treatment.J Am AcadOrthop Surg.2014;22(7): 455-464.[2] Aurégan JC,Villain B,Bégué T.What is the rate of patients undergoing a total hip arthroplasty after core decompression and insertion of a tantalum rod in osteonecrosis of the femoral head: a systematic review. IntOrthop. 2018;42(7):1631-1638.[3] Zhao DW, Hu YC.Chinese experts' consensus on the diagnosis and treatment of osteonecrosis of the femoral head in adults. Orthop Surg. 2012;4(3):125-130..[4] Kobayashi S,Kubo T,Iwamoto Y,et al.Nationwide multicenter follow-up cohort study of hip arthroplasties performed for osteonecrosis of the femoral head.IntOrthop. 2018. doi: 10.1007/s00264-018-3980-1.[5] Mont, MA,Cherian JJ,Sierra RJ,et al.Nontraumatic osteonecrosis of the femoral head: where do we stand today?: a ten-year update.J Bone Joint Surg Am.2015;97(19):1604-1627.[6] Park YS,Moon YW,Lee KH,et al.Revision hip arthroplasty in patients wth a previous total hip replacement for osteonecrosis of the femoral head.Orthopedics.2014;37(12):1058-1062.[7] Issa K,Pivec R,Kapadia BH,et al. Osteonecrosis of the femoral head: the total hip replacement solution. Bone Joint J. 2013;95-B(11 Suppl A):46-50.[8] Ishaan S, Lee YY,Peter M,et al.Common factors associated with osteonecrosis of the femoral head in young patients requiring total hip arthroplast.Hip Int.2015;25(3):232-236.[9] Luo Z,Shang X,Hu F,et al.Outcome analysis of total hip arthroplasty for traumatic avascular necrosis of femoral head.Zhonghua Yi XueZaZhi. 2014;94(23):1773-1776.[10] Zhao DW,Mang Y,Hu K,et al.Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the chinese population: results from a nationally representative survey. Chin Med J (Engl).2015;128(21):2843-2850.[11] ChotivichitA,Korwutthikulrangsri E,Pornrattanamaneewong C,et al.Core decompression with bone marrow injection for the treatment of femoral head osteonecrosis.J Med Assoc Thai.2014;97(9):139-143.[12] Li X, Xu X, Wu W.Comparison of bone marrow mesenchymal stem cells and core decompression in treatment of osteonecrosis of the femoral head: a meta-analysis.Int J Clin Exp Pathol. 2014;7(8): 5024-5030.[13] Marker DR, Seyler TM,Ulrich SD, et al.Do modern techniques improve core decompression outcomes for hip osteonecrosis?ClinOrthopRelat Res.2008;466(5):1093-1103.[14] Rajagopal M,Balch SJ,Ellis TJ. Efficacy of core decompression as treatment for osteonecrosis of the hip: a systematic review.Hip Int. 2012;22(5):489-493..[15] Zhao DW,Yu XB.Core decompression treatment of early-stage osteonecrosis of femoral head resulted from venous stasis or artery blood supply insufficiency.J Surg Res.2015;194(2):614-621.[16] Feng B,Qian WW,Weng XS,et al.Outcome of the treatment of osteonecrosis of femoral head using the core decompression with bone impaction grafting.ZhongguoYi XueKeXue Yuan Xue Bao. 2015;37(2):133-139.[17] Moya-Angeler J, Gianakos AL, Villa JC, et al. Current concepts on osteonecrosis of the femoral head. World J Orthop.2015;6(8):590-601.[18] Lim YW,Kim YS,Lee JW,et al.Stem cell implantation for osteonecrosis of the femoral head.ExpMol Med.2013:61.[19] Zhao D, Liu B, Wang B, et al. Autologous bone marrow mesenchymal stem cells associated with tantalum rod implantation and vascularized iliac grafting for the treatment of end-stage osteonecrosis of the femoral head. Biomed Res Int. 2015;2015:240506.[20] Jin HH, Xu TT, Chen QQ, et al. The fate and distribution of autologous bone marrow mesenchymal stem cells with intra-arterial infusion in osteonecrosis of the femoral head in dogs. Stem Cells Int. 2016;2016: 8616143.[21] Chen C, Qu ZG, Yin XG, et al. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: A three-year follow-up study. Mol Med Rep. 2016;14(5): 4209-4215.[22] Mao Q, Jin HT, Liao F, et al. The efficacy of targeted intraarterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: a five year follow-up study. Bone.2013;57(2):509-516.[23] Gao YS, Zhang C. Cytotherapy of osteonecrosis of the femoral head: a mini review. Int Orthop.2010; 34(6):779-782.[24] Tabatabaee RM, Saberi S, Parvizi S, et al. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty.2015;30(9):11-15.[25] Ma YC, Wang T, Liao JX, et al. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: a prospective, double-blinded, randomized, controlled study. Stem Cell Res Ther.2014; 5(5):115.[26] Persiani P,De CC,Graci J,et al.Stage-related results in treatment of hip osteonecrosis with core-decompression and autologous mesenchymal stem cells.ActaOrthop Belg.2015;81(3):406-412.[27] Nandeesh NH, Janardhan K, Subramanian V, et al. Treatment of AVN using autologous BM stem cells and activated platelet-derived growth factor concentrates. J Stem Cells.2016, 11(3):135-148.[28] Rastogi S, Sankineani SR, Nag HL, et al. Intralesional autologous mesenchymal stem cells in management of osteonecrosis of femur: a preliminary study. Musculoskelet Surg.2013;97(3): 223-228.[29] Gao YS,Chen SB, Jin DX,et al.Modified surgical techniques of free vascularized fibular grafting for treatment of the osteonecrosis of femoral head: Results from a series of 407 cases.Microsurgery. 2013;33(8):646-651.[30] Papanagiotou M,Malizos KN,Vlychou M,et al.Autologous (non-vascularised) fibular grafting with recombinant bone morphogenetic protein-7 for the treatment of femoral head osteonecrosis: preliminary report.Bone Joint J. 2014;96(1):31-35.[31] Pierce TP,Elmallah RK,Jauregui JJ,et al. A current review of non-vascularized bone grafting in osteonecrosis of the femoral head.Curr Rev Musculoskelet Med.2015;8(3):240-245.[32] Meric M, Ulusal AE, Atik A, et al. Descending branch of the lateral circumflex femoral artery as a recipient vessel for vascularized fibular grafts: Clinical case series. Microsurgery. 2014;34(8):633-637.[33] Korompilias AV, Beris AE, Lykissas MG, et al. Femoral head osteonecrosis: Why choose free vascularized fibula grafting. Microsurgery. 2011; 31(3):223.[34] Ligh CA,NelsonJA,Fischer JP,et al.The effectiveness of free vascularized fibular flaps in osteonecrosis of the femoral head and neck: a systematic review.J Reconstr Microsurg..2017;33(3):163-172.[35] Fontecha CG, Roca I, Barber I, et al. Femoral head bone viability after free vascularized fibular grafting for osteonecrosis: SPECT/CT study. Microsurgery. 2016;36(7):573-577.[36] Cao L, Guo CG, Chen JF, et al. Free vascularized fibular grafting improves vascularity compared with core decompression in femoral head osteonecrosis: a randomized clinical trial. ClinOrthopRelat Res. 2017; 475(9):2230-2240.[37] Unal MB,Cansu E, Parmaksizoglu F,et al.Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting: Results of 7.6-year follow-up.ActaOrthopTraumatolTurc.2016;50(5):501-506.[38] Zhao D, Yu XB, Wang TN, et al. Digital subtraction angiography in selection of the vascularized greater trochanter bone grafting for treatment of osteonecrosis of femoral head. Microsurgery.2013;33(8): 656-659.[39] Hu R, Lei PF, Li B, et al. Real-time computerised tomography assisted porous tantalum implant in ARCO stage I-II non-traumatic osteonecrosis of the femoral head: minimum five-year follow up. Int Orthop. 2018;42(7):1535-1544. [40] Zhang X,Wang J,Xiao J,et al. Early failures of porous tantalum osteonecrosis implants: a case series with retrieval analysis.Int Orthop.2016;40(9):1827-1834.[41] Mao Q, Wang WD, Xu TT, et al. Combination Treatment of Biomechanical Support and Targeted Intra-arterial Infusion of Peripheral Blood Stem Cells mobilized by granulocyte-colony stimulating factor for the Osteonecrosis of the Femoral Head: a randomised controlled clinical trial. J Bone Miner Res. 2015;30(4): 647-656.[42] Liu BL,Sun W,Yue DB, et al.Combined tantalum implant with bone grafting for the treatment of osteonecrosis of the femoral head.J Invest Surg.2013;26(3):158-162.[43] Zhao DW, Liu BY, Wang BJ, et al. Autologous bone marrow mesenchymal stem cells associated with tantalum rod implantation and vascularized iliac grafting for the treatment of end-stage osteonecrosis of the femoral head.Biomed Res Int.2015:240506.[44] Michio H,Yuji Y,Takuma Y, et al. The clinical and radiographic results of intertrochanteric curved varus osteotomy for idiopathic osteonecrosis of the femoral head.Arch Orthop Trauma Surg.2014;134(3):305-310.[45] Baba T, Nozawa M, Homma Y, et al. Long-term results of rotational acetabular osteotomy for osteonecrosis with collapse of the femoral head in young patients. Arch Orthop Trauma Surg. 2017;137:925-931. [46] Sonoda K, Yamamoto T, Motomura G, et al. Outcome of transtrochanteric rotational osteotomy for posttraumatic osteonecrosis of the femoral head with a mean follow-up of 12.3 years. Arch Orthop Trauma Surg. 2015;135(9):1257-1263.[47] Okura T, HasegawaY, Morita D,et al.What factors predict the failure of curved intertrochanteric varus osteotomy for the osteonecrosis of the femoral head?Arch Orthop Trauma Surg.2016;136(12):1647-1655. [48] Pyda M,Koczy B, Widuchowski W et al.Hip resurfacing arthroplasty in treatment of avascular necrosis of the femoral head. Med Sci Monit. 2015;21:304-309.[49] Lim SJ,Kim SM,Kim DW,et al.Cementless total hip arthroplasty using Biolox?delta ceramic-on-ceramic bearing in patients with osteonecrosis of the femoral head.Hip Int.2016;26(2):144-148.[50] Kim YH, Park JW, Kim JS, et al. Alumina delta-on-alumina delta bearing in cementless total hip arthroplasty in patients aged <50 years. J Arthroplasty. 2017;32(3):1048-1053.[51] Kim YH, Choi Y, Kim JS, et al. Cementless total hip arthroplasty with ceramic-on-ceramic bearing in patients younger than 45 years with femoral-head osteonecrosis.IntOrthop. 2010;34(8):1123-1127.[52] Byun JW, Yoon TR, Park KS, et al. Third-Generation ceramic-on-ceramic total hip arthroplasty in patients younger than 30 years with osteonecrosis of femoral head. J Arthroplasty. 2012;27(7): 1337-1343. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [3] | Li Dadi, Zhu Liang, Zheng Li, Zhao Fengchao. Correlation of total knee arthroplasty efficacy with satisfaction and personality characteristics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1346-1350. |
| [4] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [5] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [6] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [7] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [8] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [9] | Yuan Jiawei, Zhang Haitao, Jie Ke, Cao Houran, Zeng Yirong. Underlying targets and mechanism of Taohong Siwu Decoction in prosthetic joint infection on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1428-1433. |
| [10] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [11] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [12] | Yuan Jun, Yang Jiafu. Hemostatic effect of topical tranexamic acid infiltration in cementless total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 873-877. |
| [13] | Liu Lihua, Sun Wei, Wang Yunting, Gao Fuqiang, Cheng Liming, Li Zirong, Wang Jiangning. Type L1 steroid-induced osteonecrosis of the femoral head through femoral head and neck junction decompression by fenestration: a single-center prospective clinical study [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 906-911. |
| [14] | Li Yan, Wang Pei, Deng Donghuan, Yan Wei, Li Lei, Jiang Hongjiang. Electroacupuncture for pain control after total knee arthroplasty: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 957-963. |
| [15] | Zhao Zhongyi, Li Yongzhen, Chen Feng, Ji Aiyu. Comparison of total knee arthroplasty and unicompartmental knee arthroplasty in treatment of traumatic osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 854-859. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||