Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 145-152.doi: 10.12307/2026.508
Previous Articles Next Articles
Regulation of lysosome function by stem cells in treatment of lysosomal storage diseases
Li Yiwen1, Liu Feixiang2, Zhang Yunke1, 2
- 1First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 2Department of Encephalopathy, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450003, Henan Province, China
-
Received:2024-10-10Accepted:2024-12-31Online:2026-01-08Published:2025-07-02 -
Contact:Zhang Yunke, MD, Chief physician, Professor, Docotoral supervisor, Post-doctoral cooperative supervisor, First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; Department of Encephalopathy, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450003, Henan Province, China -
About author:Li Yiwen, Master candidate, First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China -
Supported by:National Natural Science Foundation of China, No. 81974564 (to ZYK); National Natural Science Foundation of China, No. 82104730 (to LFX); Central Plains Talent Program - Science and Technology Innovation Leading Talent Project - Henan Provincial Department of Science and Technology, No. 224200510027 (to ZYK); Henan Provincial “Double First-Class” Creation Discipline Traditional Chinese Medicine Scientific Research Special Project, No. HSRP-DFCTCM-2023-1-04 (to ZYK); Henan Provincial Traditional Chinese Medicine Scientific Research Special Project, No. 2023ZY1030 (to ZYK)
CLC Number:
Cite this article
Li Yiwen, Liu Feixiang, Zhang Yunke. Regulation of lysosome function by stem cells in treatment of lysosomal storage diseases[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 145-152.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

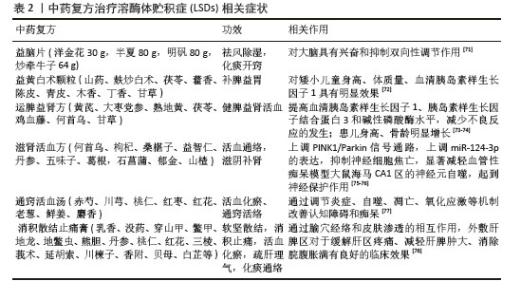
2.2 溶酶体导致LSDs的机制 溶酶体作为细胞的“消化器官”,内含丰富的酸性水解酶,能有效分解蛋白质、脂质、核酸及多糖等生物大分子,在降解和回收细胞废物、维持氨基酸和离子稳态、钙信号传导和能量代谢过程中发挥作用[13-14]。细胞内外部环境改变、刺激(溶酶体内容物积累、pH值改变、膜电位的损失以及生物、机械或化学应激源)等因素可通过触发生物发生、修复及清除过程来不断调整溶酶体的数量、大小和组成。溶酶体损伤的及时代偿和修复对于许多细胞都很重要,尤其是神经元细胞[15]。溶酶体功能对维持细胞-细胞和细胞-细胞外基质通讯、控制炎症反应和维持细胞稳态有重要意义[16];当基因突变涉及溶酶体酶、结构组分或生物发生因子的编码时,溶酶体的正常功能会受到干扰,进而影响微环境的生化特性与功能运作,包括细胞废弃物的累积、炎症反应加剧、细胞凋亡异常以及信号传导机制的失调。这些突变还可能导致溶酶体内酸性水解酶及其他关键蛋白(如激活蛋白、转运蛋白及溶酶体蛋白加工校正酶)的功能缺陷,进一步加剧病理过程[17-18]。机体中相应的生物大分子(如核酸、蛋白质、脂质、黏多糖及糖原等)降解过程受阻而在溶酶体内异常积累,进而引发细胞、组织乃至整个器官的功能障碍,这些疾病被称为LSDs,其特征是细胞分解代谢程序受损和器官功能紊乱[19-20]。溶酶体功能障碍导致神经元内部形成不溶性聚集体,这些聚集体对神经元功能产生严重影响。已观察到包括α-突触核蛋白、朊病毒蛋白、Tau蛋白和淀粉样β蛋白在内的多种淀粉样蛋白在脑部沉积,这一现象出现在各种LSDs模型中,并与多种疾病的神经病理学特征相关,可累及多个系统和器官,如神经系统、皮肤、肾脏和心脏等。因此,患者可能出现智力障碍、骨骼及神经系统发育迟缓、器官肿大、结缔组织病变以及视觉问题等多种症状[16]。溶酶体功能障碍导致部分LSDs的机制及症状见表1[21-30]。 "

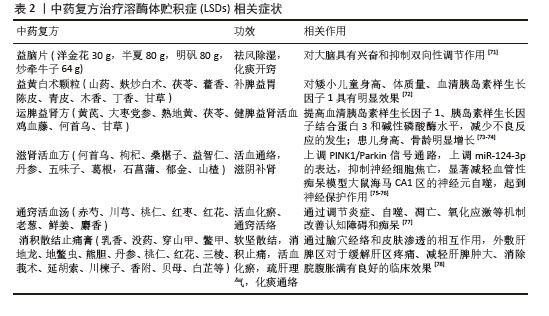
2.3 干细胞调控溶酶体功能的机制 2.3.1 干细胞分化与替代 干细胞疗法作为一种新兴的治疗方法,因独特的生物学特性而备受关注。干细胞具有自我更新和多向分化的潜能,能够分化为多种细胞类型以替代受损细胞及组织,在遗传代谢类疾病治疗中展现出巨大潜力[31]。LSD患者体内缺乏特定的溶酶体酶,导致底物在溶酶体内积累,进而引发细胞功能障碍和组织损伤。在LSDs的治疗中,干细胞可以被诱导分化为具有正常溶酶体功能的细胞,以替代病变细胞并恢复溶酶体的正常功能。已有研究表明,异体造血干细胞移植通过包括酶替代在内的多重机制为LSDs患者治疗提供了契机。作为Hurler综合征的标准治疗方案,异体造血干细胞与移植前或围移植期酶替代疗法相结合,显著提升了早期患者的总体生存率[32]。异体造血干细胞移植最早于1981 年应用于治疗Hurler 综合征并取得良好效果。将具有正常酶水平的骨髓来源细胞输注患者体内,机体以内吞途径将酶传递到溶酶体,降解细胞内贮积底物从而达到治疗疾病的目的[33]。健康的造血干细胞不仅具备在体内自我再生并分化成各类血细胞的能力,还能够在细胞外基质及血液循环内释放功能性溶酶体水解酶,这些酶随后被受体细胞摄取,从而纠正酶缺陷细胞的功能[34]。有研究显示,在7例预先接受阿仑单抗、氟达拉滨和美法仑组成的低强度、高度免疫抑制方案的Hurler 综合征患者中,有6例患儿造血功能恢复,酶水平恢复正常,存活率显著提高[35]。 2.3.2 细胞间通讯与微环境调节 干细胞能够以细胞外囊泡的方式将特定的mRNA、miRNA及蛋白质等分子,通过水平转移的方式调节受体细胞的基因表达及蛋白质合成,从而改善溶酶体的功能状态,通过细胞间通讯调节受损细胞的微环境,有效减轻炎症反应,并积极促进受损组织的修复与再生过程[36]。造血干细胞移植成功后,移植的供体造血干细胞将作为缺失酶的内源性来源,用于交叉校正有缺陷的代谢。研究表明,供体来源的巨噬细胞在穿过血脑屏障后分化为小胶质细胞,分泌缺陷酶供周围神经元重新使用[37]; 此外,供体细胞也可通过旁分泌信号传导发挥抗炎和促神经原性作用[38]。国外一项动物实验表明,小鼠侧脑室室下区的活性神经干细胞表现出溶酶体和蛋白酶体相关基因的高表达[39]。 2.3.3 调控溶酶体酶表达 基因编辑技术通过将正常基因序列导入干细胞并利用基因编辑技术纠正缺陷基因,可以获得具有正常溶酶体酶活性的干细胞,通过调控酶的产生为LSDs的基因治疗提供了新的手段。这些干细胞在分化为特定类型的细胞后,能够产生并分泌正常的溶酶体酶以补充或替代患者体内缺失的酶类。此外,干细胞还可以作为基因治疗的载体细胞,将治疗性基因递送至病变组织以实现局部治疗。例如,利用基因编辑技术改造的造血干细胞可以在体内长期存活并分化为多种血细胞类型,持续产生并分泌溶酶体酶以治疗全身性的LSDs。国外一项研究显示,干细胞治疗可通过修复基因等方式在黏多糖贮积症Ⅰ型,Hurler变体(mucopolysaccharidosis type I,Hurler variant,MPSIH)患者中广泛纠正外周及中枢神经系统的代谢异常。对8例Hurler综合征患儿实施了清髓性预处理方案后进行含α-L-艾杜糖醛酸酶(α-L-Iduronidase,IDUA)编码的慢病毒载体转导的自体造血干细胞治疗,结果均检测到基因校正细胞快速持续植入,且1个月内血液IDUA活性超生理水平,尿糖胺聚糖显著减少,4/5患儿12个月后糖胺聚糖恢复正常;脑脊液IDUA活性从无到有,这可能与糖胺聚糖局部清除有关;患儿认知、运动功能稳定,脑脊液MRI改善,关节僵硬减轻,生长正常[30]。同样有研究探索利用反转录病毒作为编码 IDUA的载体,成功地将此载体转导至骨髓间充质干细胞内,基因修饰后的骨髓间充质干细胞表达高水平的IDUA,从而使MPS-IH成纤维细胞中糖胺聚糖储存正常化;此外,基因修饰的骨髓间充质干细胞能够通过蛋白质转移交叉校正MPS-IH成纤维细胞中的酶缺陷以纠正IDUA及糖胺聚糖水平[40]。 2.4 干细胞在LSDs中应用 随着科研领域的不断深入,干细胞疗法在LSDs的治疗领域已取得了显著进展,其中,间充质干细胞与造血干细胞在尼曼-匹克病、戈谢病、黏多糖贮积症等LSDs的治疗中展现出了尤为突出的潜力和前景。 2.4.1 干细胞治疗尼曼-匹克病 鞘磷脂在溶酶体内异常积聚导致尼曼-匹克病,临床常表现为淋巴细胞浸润和内脏肿大[41]。尼曼-匹克病以对症支持治疗为主,尚无特效药物治疗。国外有研究发现,干细胞通过自我更新能力可恢复或替换被疾病破坏的细胞。基于“交叉纠正”理论[42],将健康供者的造血干细胞移植至酶缺陷患者体内可改善临床症状。国内一项研究展示了将非同源的造血干细胞移植到尼曼-匹克病患儿体内,并观察到了积极的治疗效果。该流程首先通过白消安方案的强化清髓预处理,结合全身放疗以实现免疫系统的深度清除,随后植入供者的造血干细胞,移植后第14天,FISH-XY染色体检查示供者细胞完全植活;术后半年复查鞘磷脂酶活性恢复至正常水平[43]。国外有研究者为肺部受累的尼曼-匹克病B型患者进行造血干细胞移植,移植后2个月患儿肝脾逐步恢复至正常大小,同时血清中的三酰甘油浓度与低密度脂蛋白胆固醇含量均呈现出显著下降趋势,这一变化标志着患者体内脂质代谢状况得到了有效缓解,同时间质性肺病显著改善,这与纠正酸性神经鞘磷脂酶缺陷密切相关[44]。造血干细胞移植能够显著改善多种严重脏器受累的症状,包括肝脏与脾脏的显著肿大、肝功能指标异常以及弥漫性的肺间质病变等病理状态。部分学者提出,在严重肝病及肺部疾病尚未全面显现之前实施造血干细胞移植治疗,或能为患者带来更为显著的益处,提前干预以阻止病情进一步恶化。国内有报道称尼曼匹克病B型患儿在接受外周血干细胞治疗1个月后,骨髓尼曼匹克细胞及海蓝细胞消失,移植后3个月酸性神经鞘磷脂酶活性恢复至正常水平,肝脾大小逐渐恢复正常,无肺部及神经系统受累,肝脾肋下未触及[45]。 2.4.2 干细胞治疗黏多糖贮积症 黏多糖贮积症是溶酶体病最常见的一种类型,由IDUA活性不足引起,导致糖胺聚糖积累和进行性多系统恶化,严重影响神经和肌肉骨骼系统。同种异体造血干细胞移植被认为是治疗Hurler综合征(黏多糖贮积症Ⅰ型,Hurler变异型)的金标准,可以缓解多种疾病症状并延长患者的寿命。经过长期的临床实践与验证,同种异基因造血干细胞移植可作为稳定的供应源,将酶及组织巨噬细胞递送至受影响的器官(包括大脑),从而产生比酶替代疗法更好的结果[46]。后续相关实验表明,增加循环IDUA水平可以有效增强造血干细胞移植的治疗作用。国外研究者发现移植病毒转导的小鼠造血干细胞和祖细胞在小鼠模型体内表达出超正常酶水平,使黏多糖贮积症模型小鼠运动功能得到改善,外周炎性细胞因子减少,并得出造血干细胞基因疗法单独使用或与抗炎药联合使用可能显著改善患者神经功能的结论[47]。Ⅶ型黏多糖贮积症是特发性水肿胎儿中最常见的遗传缺陷,其中大多数胎儿无法存活到出生。国外一项试验表明,在胎儿期开始造血干细胞移植可能改善LSDs患者的临床结局。与对照组相比,接受同源CX3C趋化因子受体1-绿色荧光蛋白(CX3CR1-GFP)小鼠来源造血干细胞移植的实验组小鼠存活率显著增加,这可能与移植细胞可以交叉校正其他可以摄取酶的细胞有关;进一步研究发现,供体细胞植入的程度与宿主细胞炎症标志物呈负相关,这表明造血干细胞移植可有效改善黏多糖贮积症导致的神经炎症[48]。国内同样有相关研究,5例黏多糖贮积症Ⅲ型患儿进行造血干细胞移植治疗,移植后1个月外周血白细胞酶活性均处于正常水平,移植后随访2年内智力发育稳定,较移植前未见减退,关节僵硬、骨骼畸形症状得到明显改善,因此得出造血干细胞移植治疗可以改善黏多糖贮积症Ⅲ型患儿临床症状的结论[49]。 2.4.3 干细胞治疗戈谢病 戈谢病是由于β-葡萄糖脑苷脂酶1(GBA1)基因突变引起酶缺乏,导致底物葡萄糖神经酰胺在巨噬细胞中积累,其特征是戈谢细胞(含有未裂解的葡萄糖脑苷脂的增大巨噬细胞)浸润[50]。由于治疗酶无法穿过血脑屏障,酶替代疗法难以纠正神经系统症状[51];一系列相关成果揭示了造血干细胞移植治疗在一次性纠正酶缺陷上的显著效果,不仅弥补酶替代治疗的不足,还成功实现了对进展期Ⅱ和Ⅲ型神经病变的完全稳定作用。此外,该治疗方法还显著提升了葡萄糖脑苷脂的清除效率,并完全修正了内脏与骨骼所存在的缺陷。众多病例报告均显示,造血干细胞移植能够带来显著的临床改善,包括生长发育加速、器官肿大得到缓解,甚至骨骼的病变也可能逐渐消退,控制3型戈谢病患者神经系统症状进展[52]。另有研究表明,造血干细胞移植后患者呼吸道症状在1个月内明显好转;3个月内报告胸部影像学改善、肺功能检查改善和 β-葡萄糖脑苷脂酶活性恢复正常[53]。神经性戈谢病是由β-葡萄糖神经酰胺酶遗传性缺陷引起的中枢神经系统受累,为观察造血干细胞移植是否可以逆转神经系统损伤,国外研究者通过实验观察到移植后的造血干细胞能够在骨髓及其他部位提供功能正常的巨噬细胞来源,并减轻戈谢病引起的炎症,结果表明造血干细胞移植可明显延缓神经病变进展,并改善骨骼病变[54]。近年来,国内的多项研究同样揭示了非亲属脐血造血干细胞移植的显著疗效。在脐血造血干细胞移植后,大部分患儿随着葡糖脑苷脂酶恢复正常,肝肿大和骨痛有所缓解,身高生长延迟有部分改善,并得出脐血造血干细胞移植是目前适合中国国情的治疗戈谢病的有效措施的结论[55]。 2.4.4 干细胞治疗异染性脑白质营养不良 异染性脑白质营养不良病理基础在于芳基硫酸酯酶A的功能障碍,这一缺陷促使溶酶体内脑硫脂异常积聚于中枢神经系统的白质区域及周围神经系统乃至内脏组织,进一步触发神经系统的脱髓鞘改变,最终表现为神经系统功能的持续性、进行性衰退[56]。异染性脑白质营养不良的临床表现存在个体差异,但绝大多数患者终将面临运动与认知功能的全面衰退[57]。鉴于酶类药物在穿越血脑屏障方面的局限性,酶替代疗法对于修复或缓解神经系统受损症状的效果有限。相比之下,干细胞疗法因其独特的穿越血脑屏障能力,成为当前改善异染性脑白质营养不良中枢神经系统症状的新策略。一项研究表明,间充质干细胞能够分泌芳基硫酸酯酶A,并在异染性脑白质营养不良患者中显示出有益的作用。10例儿童异染性脑白质营养不良患者接受造血干细胞移植并在移植成功后第1年内评估安全性、移植物抗宿主病的发生率、血芳基硫酸酯酶A水平、嵌合体、细胞再生和植入、MRI改变以及大体运动功能,结果表明儿童异染性脑白质营养不良患者应用间充质干细胞治疗是安全的且耐受性良好,患者的芳基硫酸酯酶A、白细胞及血小板水平显著升高,造血功能得到改善[58]。另有研究者使用慢病毒载体转导的自体造血干细胞和祖细胞疗法,结果发现在26例早发性异染性脑白质营养不良患儿中,大多数接受治疗的患儿运动功能明显改善,认知能力显著提高,中枢和外周脱髓鞘及脑萎缩进展大幅延缓;在症状出现前接受治疗的患者中,治疗益处尤为明显[59]。同样有实验证实,用慢病毒载体转导的自体造血干细胞可以规避传统移植的风险,近期的临床研究证明了其安全有效性,明显延缓病情进展[60]。 2.5 中医药治疗LSDs的研究进展 LSDs是由于溶酶体内水解酶、转运蛋白或相关酶的缺乏,导致代谢物无法有效消化而在细胞内贮积,常表现为神经系统及肝脏、脾脏等器官受累,临床常出现智力低下,运动功能减退、肌张力异常、共济失调等运动功能障碍,病变累及骨骼可出现骨骼发育不全、骨骼畸形、关节僵硬等,代谢物在肝脏和脾脏中的贮积,患者还可出现肝脾肿大等症状[61-63]。LSDs在中医上并没有直接对应的病名范畴,根据阴阳五行、脏腑经络、气血津液等理论体系,可将其归为“五迟五软”“积聚”“虚劳”等范畴,虽未有中医药治疗直接纠正溶酶体酶缺陷的报道,但可以从整体观念出发,通过中药调理、针灸等方法来改善患者的一些症状,提高身体的功能和抵抗力以达到对抗疾病的效果。周德安教授选取百会、神庭、本神、四神聪穴进行针刺,注重补先天调后天,以达到健脑益智、填精益髓的功效;针刺治疗4次后患儿运动能力加强,认知功能明显提高,治疗7次后患儿理解力明显提高[64]。针对语言发育落后,凡伟等[65]使用焦式头针和智九针联合应用语言训练,使发育迟缓儿童获得了显著的治疗效果;蒙睿等[66]采用通督开窍针法结合语言训练来治疗语言发育迟缓患儿,治疗后患儿的DQ评分均较治疗前有所提高。LSDs病变累及神经系统时常有智力低下、精神发育迟滞、生活难以自理等表现,病属“五迟”“呆病”,心脾两虚型临床较为多见,临床应用调元散治疗此类病症取得良好效果,一组随机对照试验结果显示,调元散方剂治疗组总体有效率高达90.9%,这一成效显著超越了对照组的72.7%,差异有显著性意义(P < 0.05),可见在针对心脾两虚型智力低下患者,调元散加减方相较于复方吡拉西坦脑蛋白水解物片展现出了更为优越的治疗效果[67]。在一项有关小儿脑瘫治疗的临床研究中,周旷等[68]创新性地结合了循经穴位按摩、针刺疗法与常规康复训练,结果显示,相较于仅接受常规康复训练的对照组,接受联合治疗方案的患儿粗大运动功能评估量表与日常生活活动能力量表评分均显著改善。这一发现有力支持了在常规康复训练的基础上融入循经穴位按摩与针刺疗法的综合治疗方案,能够更有效地提升脑瘫患儿的运动功能水平及日常生活自理能力。多种LSDs均可引起肝脾肿大、脾功能亢进等,可能与贮积物沉积于肝脾,影响正常生理功能有关,多种方剂如鳖甲煎丸、逍遥丸和膈下逐瘀汤等在治疗肝脾肿大方面取得明显效果。在1例婴儿肝炎综合征的临床治疗中,经验方“芽根木贼汤”合“茵陈四苓汤”取得了良好效果,三诊过后患儿肝功能恢复正常,皮肤黄染消退,肝体积缩小至正常大小,病告痊愈[69]。现代医学研究表明,中医在胆汁淤积性肝病中有多靶点的治疗优势,发挥恢复胆汁酸代谢、改善氧化应激、减轻炎症反应和抗纤维化的作用[70]。中药复方治疗LSDs相关症状详见表2[71-78]。 "
| [1] PLATT FM, D’AZZO A, DAVIDSON BL, et al. Lysosomal storage diseases. Nat Rev Dis Primers. 2018;4(1):27. [2] BOUSTANY RM. Lysosomal storage diseases--the horizon expands. Nat Rev Neurol. 2013; 9(10):583-598. [3] VAN DER PLOEG AT, REUSER AJ. Pompe’s disease. Lancet. 2008;372(9646):1342-1353. [4] MEENA NK, RABEN N. Pompe Disease: New Developments in an Old Lysosomal Storage Disorder. Biomolecules. 2020;10(9):1339. [5] PRADA CE, GRABOWSKI GA. Neuronopathic lysosomal storage diseases: clinical and pathologic findings. Dev Disabil Res Rev. 2013; 17(3):226-246. [6] EBNER M, PUCHKOV D, LÓPEZ-ORTEGA O, et al. Nutrient-regulated control of lysosome function by signaling lipid conversion. Cell. 2023;186(24):5328-5346.e26.[7] BARRAL DC, STAIANO L, GUIMAS ALMEIDA C, et al. Current methods to analyze lysosome morphology, positioning, motility and function. Traffic. 2022;23(5):238-269. [8] HANNAH WB, DERKS TGJ, DRUMM ML, et al. Glycogen storage diseases. Nat Rev Dis Primers. 2023;9(1):46. [9] LABELLA B, COTTI PICCINELLI S, RISI B, et al. A Comprehensive Update on Late-Onset Pompe Disease. Biomolecules. 2023;13(9):1279. [10] KANG L, JIN S, WANG J, et al. AAV vectors applied to the treatment of CNS disorders: Clinical status and challenges. J Control Release. 2023;355:458-473. [11] COSTI S, CAPORALI RF, MARINO A. Mucopolysaccharidosis: What Pediatric Rheumatologists and Orthopedics Need to Know. Diagnostics (Basel). 2022;13(1):75. [12] TIAN Z, YU T, LIU J, et al. Introduction to stem cells. Prog Mol Biol Transl Sci. 2023;199:3-32. [13] BALLABIO A, BONIFACINO JS. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol. 2020;21(2): 101-118. [14] YANG C, WANG X. Lysosome biogenesis: Regulation and functions. J Cell Biol. 2021; 220(6):e202102001. [15] ZHEN Y, RADULOVIC M, VIETRI M, et al. Sealing holes in cellular membranes. EMBO J. 2021;40(7):e106922. [16] SETTEMBRE C, PERERA RM. Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology. Nat Rev Mol Cell Biol. 2024;25(3):223-245. [17] PERERA RM, ZONCU R. The Lysosome as a Regulatory Hub. Annu Rev Cell Dev Biol. 2016;32:223-253. [18] SAFTIG P, KLUMPERMAN J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10(9):623-635. [19] BONAM SR, WANG F, MULLER S. Lysosomes as a therapeutic target. Nat Rev Drug Discov. 2019;18(12):923-948. [20] MEDINA DL. Lysosomal calcium and autophagy. Int Rev Cell Mol Biol. 2021;362:141-170. [21] GERMAIN DP, ALTARESCU G, BARRIALES-VILLA R, et al. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol Genet Metab. 2022;137(1-2):49-61. [22] LENDERS M, BRAND E. Fabry Disease: The Current Treatment Landscape. Drugs. 2021; 81(6):635-645. [23] WANG A, CHEN C, MEI C, et al. Innate immune sensing of lysosomal dysfunction drives multiple lysosomal storage disorders. Nat Cell Biol. 2024;26(2):219-234. [24] GARCÍA MORALES L, MUSTELIER BÉCQUER RG, PÉREZ JOGLAR L, et al. Sandhoff disease in the elderly: a case study. Amyotroph Lateral Scler Frontotemporal Degener. 2022;23(1-2): 137-138. [25] KAYA E, SMITH DA, SMITH C, et al. Beneficial Effects of Acetyl-DL-Leucine (ADLL) in a Mouse Model of Sandhoff Disease. J Clin Med. 2020; 9(4):1050. [26] VITNER EB, SALOMON R, FARFEL-BECKER T, et al. RIPK3 as a potential therapeutic target for Gaucher’s disease. Nat Med. 2014;20(2): 204-208. [27] LIM JA, LI L, RABEN N. Pompe disease: from pathophysiology to therapy and back again. Front Aging Neurosci. 2014;6:177. [28] SIMON MJ, LOGAN T, DEVOS SL, et al. Lysosomal functions of progranulin and implications for treatment of frontotemporal dementia. Trends Cell Biol. 2023;33(4): 324-339. [29] FESTA BP, BERQUEZ M, NIERI D, et al. Endolysosomal Disorders Affecting the Proximal Tubule of the Kidney: New Mechanistic Insights and Therapeutics. Rev Physiol Biochem Pharmacol. 2023;185:233-257. [30] GENTNER B, TUCCI F, GALIMBERTI S, et al. Hematopoietic Stem- and Progenitor-Cell Gene Therapy for Hurler Syndrome. N Engl J Med. 2021;385(21):1929-1940. [31] KARAGIANNIS P, TAKAHASHI K, SAITO M, et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol Rev. 2019;99(1):79-114. [32] PARINI R, DEODATO F, DI ROCCO M, et al. Open issues in Mucopolysaccharidosis type I-Hurler. Orphanet J Rare Dis. 2017; 12(1):112. [33] HOBBS JR, HUGH-JONES K, BARRETT AJ, et al. Reversal of clinical features of Hurler’s disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet. 1981; 2(8249):709-712. [34] TAN EY, BOELENS JJ, JONES SA, et al. Hematopoietic Stem Cell Transplantation in Inborn Errors of Metabolism. Front Pediatr. 2019;7:433. [35] HANSEN MD, FILIPOVICH AH, DAVIES SM, et al. Allogeneic hematopoietic cell transplantation (HCT) in Hurler’s syndrome using a reduced intensity preparative regimen. Bone Marrow Transplant. 2008;41(4):349-353. [36] HARRELL CR, FELLABAUM C, JOVICIC N, et al. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019; 8(5):467. [37] ALDENHOVEN M, KURTZBERG J. Cord blood is the optimal graft source for the treatment of pediatric patients with lysosomal storage diseases: clinical outcomes and future directions. Cytotherapy. 2015;17(6):765-774. [38] KRIVIT W, SUNG JH, SHAPIRO EG, et al. Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transplant. 1995;4(4):385-392. [39] LEEMAN DS, HEBESTREIT K, RUETZ T, et al. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science. 2018;359(6381): 1277-1283. [40] BAXTER MA, WYNN RF, DEAKIN JA, et al. Retrovirally mediated correction of bone marrow-derived mesenchymal stem cells from patients with mucopolysaccharidosis type I. Blood. 2002;99(5):1857-1859. [41] KOLODNY EH. Niemann-Pick disease. Curr Opin Hematol. 2000;7(1):48-52. [42] STEINBERG SJ, MONDAL D, FENSOM AH. Co-cultivation of Niemann-Pick disease type C fibroblasts belonging to complementation groups alpha and beta stimulates LDL-derived cholesterol esterification. J Inherit Metab Dis. 1996;19(6):769-774. [43] 潘静,耿哲,江华,等.异基因造血干细胞移植治疗儿童尼曼匹克病1例报告[J].中国当代儿科杂志,2013,15(9):782-784. [44] QUARELLO P, SPADA M, PORTA F, et al. Hematopoietic stem cell transplantation in Niemann-Pick disease type B monitored by chitotriosidase activity. Pediatr Blood Cancer. 2018;65(2):e26811. [45] 陈姣,刘小梅,肖娟,等.异基因造血干细胞移植治疗尼曼匹克病B型1例[J].中国小儿血液与肿瘤杂志,2021,26(1):48-50. [46] WYNN RF, WRAITH JE, MERCER J, et al. Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J Pediatr. 2009;154(4):609-611. [47] HOLLEY RJ, ELLISON SM, FIL D, et al. Macrophage enzyme and reduced inflammation drive brain correction of mucopolysaccharidosis IIIB by stem cell gene therapy. Brain. 2018;141(1):99-116. [48] NGUYEN QH, WITT RG, WANG B, et al. Tolerance induction and microglial engraftment after fetal therapy without conditioning in mice with Mucopolysaccharidosis type VII. Sci Transl Med. 2020;12(532):eaay8980. [49] 刘军,刘芳,乔广明.黏多糖贮积症Ⅲ型基因突变谱及造血干细胞移植效果分析[J].现代中西医结合杂志,2023,32(19):2712-2716. [50] STIRNEMANN J, BELMATOUG N, CAMOU F, et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int J Mol Sci. 2017;18(2):441. [51] SCHIFFMANN R, SEVIGNY J, ROLFS A, et al. The definition of neuronopathic Gaucher disease. J Inherit Metab Dis. 2020;43(5):1056-1059. [52] SOMARAJU UR, TADEPALLI K. Hematopoietic stem cell transplantation for Gaucher disease. Cochrane Database Syst Rev. 2017; 10(10):CD006974. [53] LEE FS, YEN HJ, NIU DM, et al. Allogeneic hematopoietic stem cell transplantation for treating severe lung involvement in Gaucher disease. Mol Genet Metab Rep. 2020;25: 100652. [54] DONALD A, BJÖRKVALL CK, VELLODI A, et al. Thirty-year clinical outcomes after haematopoietic stem cell transplantation in neuronopathic Gaucher disease. Orphanet J Rare Dis. 2022;17(1):234. [55] 唐湘凤,卢伟,井远方,等.非血缘脐血或单倍体来源的造血干细胞移植治疗戈谢病的临床研究[J].中国小儿血液与肿瘤杂志, 2020,25(4):195-199. [56] GREENFIELD JG. A Form of progressive cerebral sclerosis in infants associated with primary degeneration of the interfascicular glia. J Neurol Psychopathol. 1933;13(52):289-302. [57] 陈丽,王静敏.异染性脑白质营养不良的研究进展[J].生物化学与生物物理进展,2022, 49(11): 2107-2114. [58] CABANILLAS STANCHI KM, BÖHRINGER J, STRÖLIN M, et al. Hematopoietic Stem Cell Transplantation with Mesenchymal Stromal Cells in Children with Metachromatic Leukodystrophy. Stem Cells Dev. 2022;31(7-8):163-175. [59] FUMAGALLI F, CALBI V, NATALI SORA MG, et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet. 2022;399(10322):372-383. [60] BRADBURY AM, REAM MA. Recent Advancements in the Diagnosis and Treatment of Leukodystrophies. Semin Pediatr Neurol. 2021;37:100876. [61] 张尧,熊晖.溶酶体贮积症的骨改变[J].中国实用儿科杂志,2022,37(8):613-618. [62] 郝美美,邓艳春,张彦.溶酶体贮积症导致的神经系统损害[C]//CAAE第五届国际癫痫论坛组委会.第五届CAAE国际癫痫论坛论文集.重庆:第四军医大学西京医院神经内科,2013. [63] 刘誉,吴彬彬,伍小华,等.溶酶体贮积症的分子生物学机制[J].暨南大学学报(自然科学与医学版),2013,34(4):359-366. [64] 徐俊峰,杨远滨,许世闻,等.周德安针灸治神理论在小儿发育迟缓中的应用[J].北京中医药,2018,37(3):230-231. [65] 凡伟,王容,邓磊,等.焦氏头针配合智九针早期治疗精神发育迟滞患儿的临床疗效研究[J].中国儿童保健杂志,2017,25(7):740-742. [66] 蒙睿,李芋均,罗茜,等.儿童语言发育迟缓的中医治疗研究进展[J].医学信息,2022, 35(19): 152-154. [67] 刘玉堂,贾建真,陈冬梅,等.调元散加减治疗心脾两虚型智力低下疗效观察[J].西部中医药,2018,31(11):75-77. [68] 周旷,黎治荣.循经穴位按摩+针刺联合康复训练治疗小儿脑瘫随机平行对照研究[J].实用中医内科杂志,2017,31(11):63-65. [69] 任军芳.中医药治疗婴儿肝炎综合征的体会[J].陕西中医学院学报,2001,24(2):21. [70] WEI C, QIU J, WU Y, et al. Promising traditional Chinese medicine for the treatment of cholestatic liver disease process (cholestasis, hepatitis, liver fibrosis, liver cirrhosis). J Ethnopharmacol. 2022;297:115550. [71] 王粟实,赵安业,王红伟,等.马若飞中医药治疗小儿脑瘫临床经验[J].中华中医药杂志,2024,39(7):3492-3500. [72] 闵晓雪,莫愁,龙宏.益黄白术颗粒治疗儿童偏矮小临床观察[J].中国中医药现代远程教育,2024,22(5):108-110. [73] 刘应科,崔红,杨健,等.中医药治疗儿科领域临床优势病种的探讨[J].中国实验方剂学杂志,2024,30(15):224-231. [74] 刘锋,杨雯轩,刘乾生,等.矮身材儿童中医治疗研究进展[J].中国中西医结合儿科学,2020,12(2):128-131. [75] ZHAO Z, XIE L, SHI J, et al. Neuroprotective Effect of Zishen Huoxue Decoction treatment on Vascular Dementia by activating PINK1/Parkin mediated Mitophagy in the Hippocampal CA1 Region. J Ethnopharmacol. 2024;319(Pt 1):117172. [76] YAO T, XIE L, XIE Y, et al. Protective effects of Zishen Huoxue recipe against neuronal injury in the neurovascular unit of rats with vascular dementia by interfering with inflammatory cascade-induced pyroptosis. Neuropeptides. 2023;102:102358. [77] 李东东,陈晶.通窍活血汤治疗血管性认知障碍和痴呆的研究进展[J].中华中医药学刊, 2024,42(10):103-108. [78] 朱岩洁.消积散结止痛膏治疗肝硬化肝脾肿大的临床疗效观察[D].郑州:河南中医药大学,2017. |
| [1] | Liu Xinyue, Li Chunnian, Li Yizhuo, Xu Shifang. Regeneration and repair of oral alveolar bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1247-1259. |
| [2] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [3] | Chen Qiheng, Weng Tujun, Peng Jiang. Effect of dimethylglyoxal glycine on osteogenic, adipogenesis differentiation, and mitophagy of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 50-57. |
| [4] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [5] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [6] | . Effect of miR-26b on neural and vascular differentiation in stem cells from human exfoliated deciduous teeth [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7769-7775. |
| [7] | Zhang Min, Zhang Nini, Huang Guilin, Li Zhuangzhuang, Wang Xue, Wang Huike. Human amniotic mesenchymal stem cell exosomes repair radiation-induced submandibular gland damage in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7804-7815. |
| [8] | Han Mengjun, Xu Fang. Hematopoietic stem cell mobilization: advantages and disadvantages of different plans and improvements in predictive models and technologies [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7863-7871. |
| [9] | Li Zhe, Li Ping, Zhang Chao, Guo Guangling. A network meta-analysis of efficacy of mesenchymal stem cells from different sources in treatment of premature ovarian failure animal models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7898-7908. |
| [10] | Jiang Qiyu, Zeng Huiyan. A novel analysis and prediction method for potential mechanisms of traditional Chinese medicine based on artificial intelligence and omics data-driven approach [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7552-7561. |
| [11] | Zhu Chuanxi, Qiu Long, Li Lingxu, Ji Guangcheng. Chinese herbal prescription combined with head acupuncture exercise therapy improves limb spasticity in rats with ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7571-7577. |
| [12] | Zhu Jiaping, Gao Bo, Lou Chunbiao, Yang Fengyong, Yang Kun. Monomeric traditional Chinese medicine in the treatment of rheumatoid arthritis: regulation of T cell balance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6955-6962. |
| [13] | Zhang Pulian, Liu Baoru, Yang Min . Mesenchymal stem cells for treatment of aplastic anemia: inhibiting or activating relevant targets in its pathological evolution [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6800-6810. |
| [14] | Xu Zhenhua, Li Yanjie, Qin Hewei, Liu Haoyuan, Zhu Bochao, Wang Yupu. Traditional Chinese medicine monomer in treatment of neuroinflammation after spinal cord injury: effects of nuclear transcription factor kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 590-598. |
| [15] | Liu Hanfei, Cai Zhencun, Zhou Xueting, Wen Hang, Chen Zhenjun. Mechanisms by which traumatic brain injury promotes bone callus formation and fracture healing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6260-6268. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

