Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (1): 1-7.doi: 10.12307/2024.733
Oral Herombopag Olamine and subcutaneous recombinant human thrombopoietin after haploidentical hematopoietic stem cell transplantation
Kong Dai, Wang Xinkai, Zhang Wenhui, Pei Xiaohang, Lian Cheng, Niu Xiaona, Guo Honggang, Niu Junwei, Zhu Zunmin, Liu Zhongwen
- Department of Hematology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China
-
Received:2023-10-17Accepted:2023-11-21Online:2025-01-08Published:2024-05-17 -
Contact:Liu Zhongwen, MD, Chief physician, Department of Hematology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China -
About author:Kong Dai, Master, Attending physician, Department of Hematology, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China -
Supported by:Provincial and Ministerial Joint Project of Henan Provincial Medical Science and Technology Project, No. SBGJ202102041 (to LZW); Joint Project of Henan Provincial Medical Science and Technology Project, No. LHGJ20230016 (to ZWH)
CLC Number:
Cite this article
Kong Dai, Wang Xinkai, Zhang Wenhui, Pei Xiaohang, Lian Cheng, Niu Xiaona, Guo Honggang, Niu Junwei, Zhu Zunmin, Liu Zhongwen. Oral Herombopag Olamine and subcutaneous recombinant human thrombopoietin after haploidentical hematopoietic stem cell transplantation[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 1-7.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
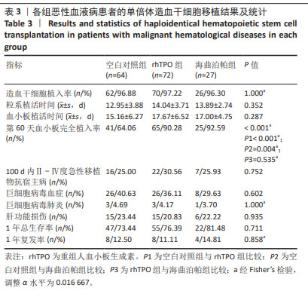
2.4 造血重建情况 经植活鉴定,空白对照组、rhTPO 组 及海曲泊帕组分别有 2 例、2 例、1 例出现原发植入失败, 植 入 成 功 率 分 别 为 96.88%,97.22%,96.30%, 差 异无显著性意义 (P=1.000)。中性粒细胞植入时间分别为 (12.95±3.88) d、(14.04±3.71) d、(13.89±2.74) d, 差 异 无 显著性意义 (P=0.352);血小板植入时间分别为 (15.16± 6.27) d、(17.67±6.52) d、(17.00±4.75) d,差异无显著性意 义 (P=0.287)。第 60 天对血小板完全植入率进行统计, 空白对照组、rhTPO 组及海曲泊帕组分别为 64.06%, 90.28%,92.59%,差异有显著性意义 (P < 0.001),随后进 行亚组分析,空白对照组与 rhTPO 组比差异有显著性意 义 (P < 0.001),空白对照组与海曲泊帕组比差异有显著性 意义 (P=0.004),rhTPO 组与海曲泊帕组比差异无显著性意 义 (P=0.535),见表 3。 2.5 急性移植物抗宿主病发生率 对 100 d Ⅱ - Ⅳ度急 性移植物抗宿主病发生率进行统计分析,空白对照组、 rhTPO 组、海曲泊帕组分别为 25.00%,30.56%,25.93%, 差异无显著性意义 (P=0.752),见表 3。"
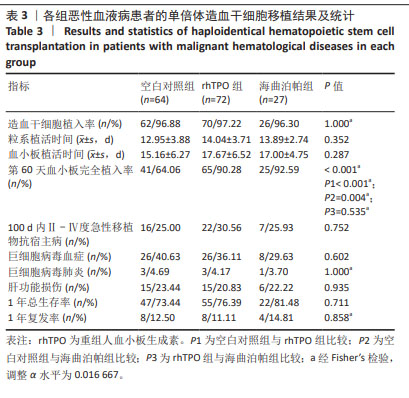
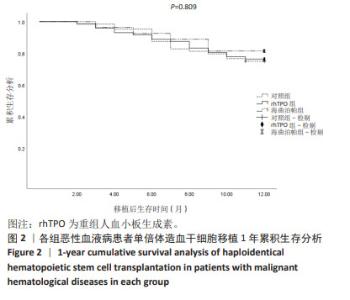
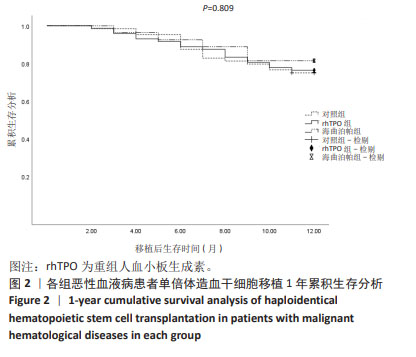
2.6 巨细胞病毒感染及巨细胞病毒肺炎发生情况 对随 访期内巨细胞病毒血症发生率进行统计,空白对照组、 rhTPO 组、海曲泊帕组分别为 40.63%,36.11%,29.63%, 差异无显著性意义 (P=0.602);3 组分别有 3 例、3 例、1 例患者进展为巨细胞病毒肺炎,发生率为 4.69%,4.17%, 3.70%,差异无显著性意义 (P=1.000),见表 3。 2.7 肝功能损伤发生率 对 3 组患者 30 d 内肝功能损伤 进行统计分析,空白对照组、rhTPO 组、海曲泊帕组发生 率分别为 23.44%,20.83%,22.22%,差异无显著性意义 (P=0.935),均为Ⅰ - Ⅱ度肝功能损伤,经应用保肝药物后 实验室指标异常迅速消失,对后续用药未造成影响,见 表 3。 2.8 血栓 随访期内,3 组患者均无血栓事件发生。 2.9 复发与生存情况 随访 1 年节点对 3 组患者的生存率 进行统计分析,空白对照组、rhTPO 组、海曲泊帕组分别 为 73.44%,76.39%,81.48%,差异无显著性意义 (P=0.711)。 随访 1 年节点对 3 组患者的复发率进行统计分析,空白对 照组、rhTPO 组、海曲泊帕组分别为 12.50%,11.11%, 14.81%,差异无显著性意义 (P=0.858)。对 3 组患者 1 年 累积生存率进行 Kaplan-Meier 分析,差异无显著性意义 (P=0.809),见表 3,图 2。 "
| [1] YAMAZAKI R, KUWANA M, MORI T, et al. Prolonged thrombocytopenia
affer allogeneic hematopoiettc stem cell transplantatton: associattons
with impaired platelet productton and increased platelet turnover.
Bone Marrow Transplant. 2006;38(5):377-384. [2] HUANG AJ, HU XX, WANG JM. Research Progress on Thrombocytopenia affer Allogeneic Hematopoiettc Stem Cell Transplantatton -Review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017; 25(1):270-275. [3] HUANG XJ. Current status of haploidenttcal stem cell transplantatton for leukemia. J Hematol Oncol. 2008;1:27. [4] KANDA Y, CHIBA S, HIRAI H, et al. Allogeneic hematopoiettc stem cell transplantatton from family members other than HLA-identtcal siblings over the last decade (1991-2000). Blood. 2003;102(4): 1541-1547. [5] KUZMINA Z, EDER S, BÖHM A, et al. Signiffcantly worse survival of pattents with NIH-deffned chronic graff-versus-host disease and thrombocytopenia or progressive onset type: results of a prospecttve study. Leukemia. 2012;26(4):746-756. [6] ALEXANDER WS, ROBERTS AW, NICOLA NA, et al. Deffciencies in progenitor cells of multtple hematopoiettc lineages and defecttve megakaryocytopoiesis in mice lacking the thrombopoiettc receptor c-Mpl. Blood. 1996;87(6):2162-2170. [7] HAN TT, XU LP, LIU DH, et al. Recombinant human thrombopoiettn promotes platelet engraffment affer haploidenttcal hematopoiettc stem cell transplantatton: a prospecttve randomized controlled trial. Ann Hematol. 2015;94(1):117-128. [8] BUSSEL JB, SOFF G, BALDUZZI A, et al. A Review of Romiplosttm Mechanism of Actton and Clinical Applicability. Drug Des Devel Ther. 2021;15:2243-2268. [9] WEN B, ZHANG X, CHEN S, et al. Oral eltrombopag versus subcutaneous recombinant human thrombopoiettn for promottng platelet engraffment affer allogeneic stem cell transplantatton: A prospecttve, non-inferiority, randomized controlled trial. Hematol Oncol. 2022;40(4):777-786. [10] MEI H, LIU X, LI Y, et al. A multtcenter, randomized phase III trial of hetrombopag: a novel thrombopoiettn receptor agonist for the treatment of immune thrombocytopenia. J Hematol Oncol. 2021; 14(1):37. [11] ZHANG F, PENG G, HE G, et al. A Multtcenter, Open-Label, Single-Arm, Phase 2 Study to Evaluate the Efffcacy and Safety of Hetrombopag in Pattents with Severe Aplasttc Anemia (SAA). Blood. 2020;136:3-4. [12] XIE C, ZHAO H, BAO X, et al. Pharmacological characterizatton of hetrombopag, a novel orally acttve human thrombopoiettn receptor agonist. J Cell Mol Med. 2018;22(11):5367-5377. [13] 孔黛 , 陈香丽 , 裴晓杭 , 等 . 去除第 11 天甲氨蝶呤对单倍体造血干 细胞移植患者发生急性移植物抗宿主病的影响 [J]. 中国组织工程 研究 ,2022,26(31):5026-5031. [14] SCHOEMANS HM, LEE SJ, FERRARA JL, et al. EBMT-NIH-CIBMTR Task Force positton statement on standardized terminology & guidance for graff-versus-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401-1415. [15] FORMAN SJ, NEGRIN RS, ANTIN JH, et al. Thomas’ Hematopoiettc Cell Transplantatton: Stem Cell Transplantatton[M]. 5th Ed. West Sussex, UK: John Wiley & Sons, Ltd. 2015:1012-1019. [16] ALSHEMMARI S, AMEEN R, GAZIEV J. Haploidenttcal hematopoiettc stem-cell transplantatton in adults. Bone Marrow Res. 2011;2011: 303487. [17] BLUMBERG N, HEAL JM, PHILLIPS GL, et al. Platelets--to transfuse or not to transfuse. Lancet. 2012;380(9850):1287-1289. [18] ZHANG X, FU H, XU L, et al. Prolonged thrombocytopenia following allogeneic hematopoiettc stem cell transplantatton and its associatton with a reductton in ploidy and an immaturatton of megakaryocytes. Biol Blood Marrow Transplant. 2011;17(2):274-280. [19] MARSH JC, MUFTI GJ. Eltrombopag: a stem cell cookie? Blood. 2014; 123(12):1774-1775. [20] KUTER DJ. New thrombopoiettc growth factors. Blood. 2007;109(11): 4607-4616. [21] COHN CS, BUSSEL JB. Romiplosttm: a second-generatton thrombopoiettn agonist. Drugs Today (Barc). 2009;45(3):175-188. [22] KAPUR R, ASLAM R, SPECK ER, et al. Thrombopoiettn receptor agonist (TPO-RA) treatment raises platelet counts and reduces antt-platelet anttbody levels in mice with immune thrombocytopenia (ITP). Platelets. 2020;31(3):399-402. [23] POLVERELLI N, CATANI L, SOLLAZZO D, et al. Platelet ffuctuattons during thrombopoiettn-receptor agonist treatment: correlatton with platelet apoptosis. Ann Hematol. 2015;94(2):339-341. [24] CHINESE SOCIETY OF HEMATOLOGY, CHINESE MEDICAL ASSOCIATION. Chinese expert consensus on the management of hemorrhagic complicattons affer hematopoiettc stem cell transplantatton(2021). Zhonghua Xue Ye Xue Za Zhi. 2021;42(4):276-280. [25] AHMED S, BASHIR Q, BASSETT R, et al. Eltrombopag for PostTransplantatton Thrombocytopenia: Results of Phase II Randomized, Double-Blind, Placebo-Controlled Trial. Transplant Cell Ther. 2021;27(5): 430.e1-430.e7. [26] LIESVELD JL, PHILLIPS GL 2ND, BECKER M, et al. A phase 1 trial of eltrombopag in pattents undergoing stem cell transplantatton affer total body irradiatton. Biol Blood Marrow Transplant. 2013;19(12): 1745-1752. [27] CATALÁ-LÓPEZ F, CORRALES I, DE LA FUENTE-HONRUBIA C, et al. Risk of thromboembolism with thrombopoiettn receptor agonists in adult pattents with thrombocytopenia: Systemattc review and metaanalysis of randomized controlled trials. Med Clin (Barc). 2015;145(12): 511-519. [28] MITTELMAN M, PLATZBECKER U, AFANASYEV B, et al. Eltrombopag for advanced myelodysplasttc syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. Lancet Haematol. 2018;5(1):e34-e43. [29] PEFFAULT DE LATOUR R, KULASEKARARAJ A, IACOBELLI S, et al. Eltrombopag Added to Immunosuppression in Severe Aplasttc Anemia. N Engl J Med. 2022;386(1):11-23. [30] WOLFF SN, HERZIG R, LYNCH J, et al. Recombinant human thrombopoiettn (rhTPO) affer autologous bone marrow transplantatton: a phase I pharmacokinettc and pharmacodynamic study. Bone Marrow Transplant. 2001;27(3):261-268. [31] ZHENG X, ZHANG H, GUO W, et al. Herombopag promotes platelet engraffment and decreases platelet transfusion affer allogeneic hematopoiettc stem cell transplantatton. Eur J Haematol. 2023;110(5):527-533. [32] GHANIMA W, JUNKER P, HASSELBALCH HC, et al. Fibroproliferattve acttvity in pattents with immune thrombocytopenia (ITP) treated with thrombopoiettc agents. Br J Haematol. 2011;155(2):248-255. [33] GHANIMA W, GEYER JT, LEE CS, et al. Bone marrow ffbrosis in 66 pattents with immune thrombocytopenia treated with thrombopoiettnreceptor agonists: a single-center, long-term follow-up. Haematologica. 2014;99(5):937-944. [34] MOUSTAFA I, FETOUH SM, BADAWY ME. Eltrombopag-Induced Myeloffbrosis in Pattents with Adult Immune Thrombocytopenia: Scoping Review. EMJ Hematol. 2019;7(1):69-79. [35] GHANIMA W, COOPER N, RODEGHIERO F, et al. Thrombopoiettn receptor agonists: ten years later. Haematologica. 2019;104(6): 1112-1123. |
| [1] | Zhang Pulian, Liu Baoru, Yang Min . Mesenchymal stem cells for treatment of aplastic anemia: inhibiting or activating relevant targets in its pathological evolution [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6800-6810. |
| [2] | Wen Jianyun, Chen Libai, He Yuelin, Feng Xiaoqin, Liu Xuan, Xu Xiaoxiao, Li Xiu, Liu Qiujun, Wu Xuedong. Human leukocyte antigen matched sibling fresh cord blood transplantation for beta-thalassaemia major in children [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4899-4906. |
| [3] | Xia Mengting, Sun Runjie, Fu Jiaqi, Li Suzhen, Yu Manya, Cui Xing. Mechanism by which Huangqintang regulates intestinal flora for treatment of intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 95-102. |
| [4] | Tu Meijuan, Zhang Chunli, Deng Li, Fang Bing, Sun Guangyu, Zhu Xiaoyu, Zhang Xinqiong. Characteristics of acute graft-versus-host disease of the intestine after unrelated cord blood transplantation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 3955-3959. |
| [5] | Zhang Jinghua, Bai Lijing, Yu Chunmei. Effect of Zishen-Yutai pills on refrozen-thawed embryo transfer in patients with diminished ovarian reserve [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 3037-3041. |
| [6] | Gao Siyu, Yao Lihong, Bian Zhilei, Zhang Suping, Li Li, Fan Jinpeng, Qin Jing, Peng Yingnan, Wan Dingming. Analysis of risk factors for short-term death after allogeneic hematopoietic stem cell transplantation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2009-2016. |
| [7] | Zhang Wenhui, Pei Xiaohang, Kong Dai, Liu Zhongwen. Efficacy of umbilical cord mesenchymal stem cells replacing donor bone marrow cells in haploidentical hematopoietic stem cell transplantation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2042-2046. |
| [8] | Peng Yingnan, Bian Zhilei, Zhang Suping, Li Li, Cao Weijie, Wan Dingming. Effect of CD34+ cell dose on haploidentical hematopoietic stem cell transplantation for treating malignant hematological diseases [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 1-6. |
| [9] | Gao Wenyi, Zhang Dong, Li Caixia, Du Juan, Zhang Yanru, Deng Yun. Interpretation of the embryo laboratory: number of oocytes retrieved affects the pregnancy outcomes of in vitro fertilization [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3871-3876. |
| [10] | Huang Chuwen, Jiang Hua, Li Minqing. Complications and death causes of peripheral blood stem cell transplantation in the treatment of thalassemia major [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 42-48. |
| [11] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [12] | Kong Dai, Chen Xiangli, Pei Xiaohang, Niu Xiaona, Chen Yuqing, Zhu Zunmin, Lei Pingchong, Sun Kai, Liu Zhongwen. Omission of day 11 methotrexate in regimen for the prophylaxis of graft-versus-host disease after haploid hematopoietic stem cell transplantation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 5026-5031. |
| [13] | Wen Jianyun, Miao Lili, Guan Di, Liu Xuan, Chen Libai, Feng Xiaoqin, Xu Xiaoxiao, Liu Qiujun, Wu Xuedong, He Yuelin. Correlation of the level of plasma biomarkers with acute graft-versus-host disease in children [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 5032-5039. |
| [14] | Yu Manya, Kong Fansheng, Zhang Jie, Xu Jie, Cui Xing. Pathogenesis of intestinal acute graft-versus-host disease after haploid hematopoietic stem cell transplantation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4806-4811. |
| [15] | Xu Anhui, Lin Xiuxiu, Zhang Xuhan, Song Kaidi, Sun Guangyu, Tang Baolin, Sun Zimin, Zheng Changcheng. Mechanism of separation of graft-versus-host disease and graft versus leukemia in unrelated cord blood transplantation for hematological malignancies [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(13): 1993-1999. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||