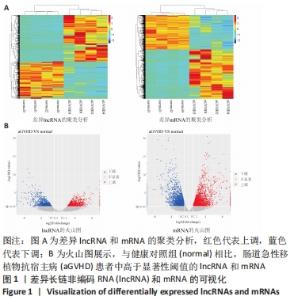
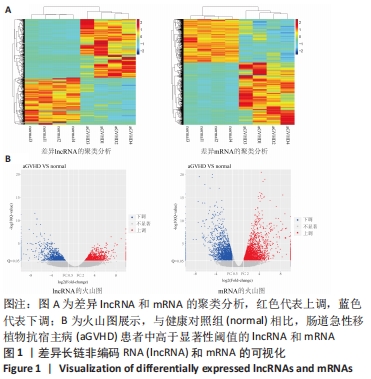
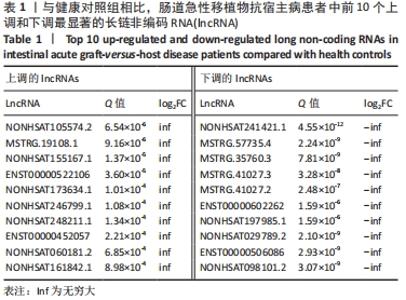
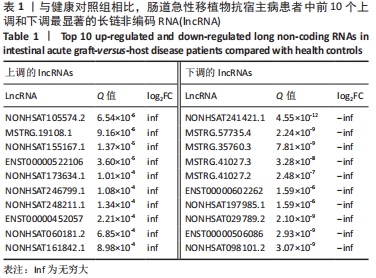
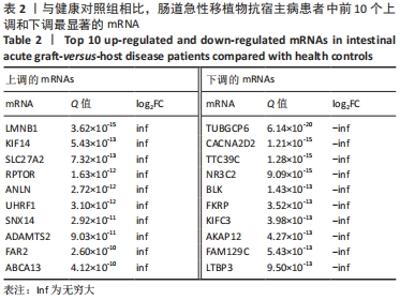
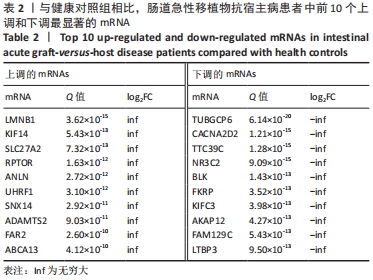
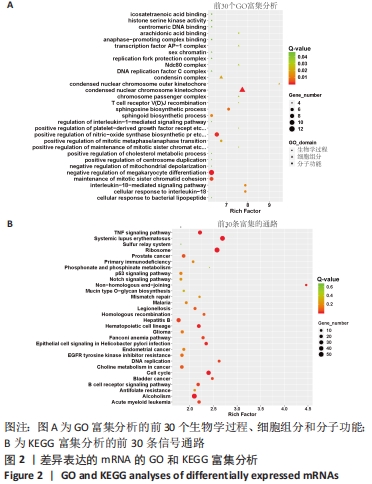
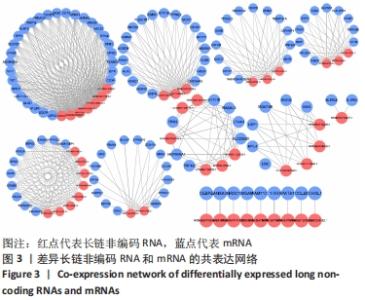
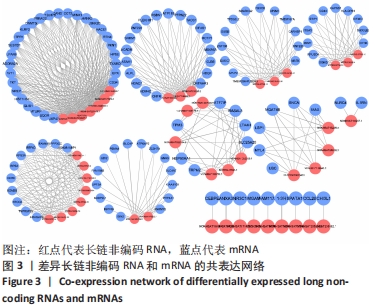
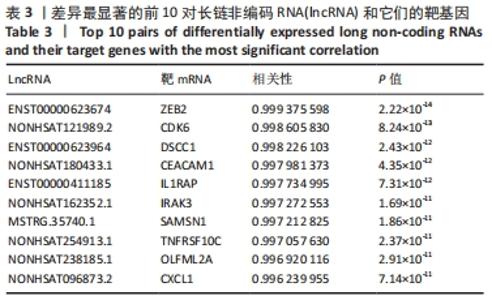
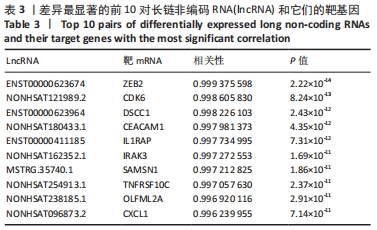
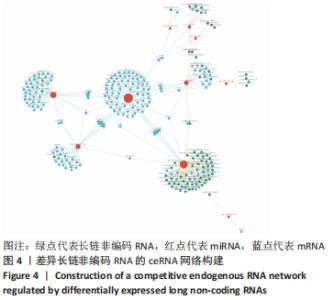
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (30): 4806-4811.doi: 10.12307/2022.735
Previous Articles Next Articles
Pathogenesis of intestinal acute graft-versus-host disease after haploid hematopoietic stem cell transplantation
Yu Manya1, Kong Fansheng2, Zhang Jie2, Xu Jie2, Cui Xing2
- 1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 2Department of Hematology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250011, Shandong Province, China
-
Received:2021-11-18Accepted:2021-12-16Online:2022-10-28Published:2022-03-29 -
Contact:Cui Xing, MD, Chief physician, Master’s supervisor, Department of Hematology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250011, Shandong Province, China -
About author:Yu Manya, Master candidate, College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
Supported by:General Project of National Natural Science Foundation of China, No. 81774080 (to CX); Taishan Scholars Young Expert Talent Project, No. tsqn201812145 (to CX)
CLC Number:
Cite this article
Yu Manya, Kong Fansheng, Zhang Jie, Xu Jie, Cui Xing. Pathogenesis of intestinal acute graft-versus-host disease after haploid hematopoietic stem cell transplantation[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4806-4811.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] BLAZAR BR, MURPHY WJ, ABEDI M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443-458. [2] MARKEY KA, MACDONALD KP, HILL GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124(3):354-362. [3] SHLOMCHIK WD. Graft-versus-host disease. Nat Rev Immunol. 2007; 7(5):340-352. [4] FERRARA JL, LEVINE JE, REDDY P, et al. Graft-versus-host disease. Lancet. 2009;373(9674):1550-1561. [5] FANG H, BONORA G, LEWANDOWSKI JP, et al. Trans- and cis-acting effects of Firre on epigenetic features of the inactive X chromosome. Nat Commun. 2020;11(1):6053. [6] NIE J, ZHAO Q. Lnc-ITSN1-2, Derived From RNA Sequencing, Correlates With Increased Disease Risk, Activity and Promotes CD4+ T Cell Activation, Proliferation and Th1/Th17 Cell Differentiation by Serving as a ceRNA for IL-23R via Sponging miR-125a in Inflammatory Bowel Disease. Front Immunol. 2020;11:852. [7] RANZANI V, ROSSETTI G, PANZERI I, et al. The long intergenic noncoding RNA landscape of human lymphocytes highlights the regulation of T cell differentiation by linc-MAF-4. Nat Immunol. 2015;16(3):318-325. [8] XIA M, LIU J, LIU S, et al. Ash1l and lnc-Smad3 coordinate Smad3 locus accessibility to modulate iTreg polarization and T cell autoimmunity. Nat Commun. 2017;8:15818. [9] TANAKA J, IMAMURA M, KASAI M, et al. Cytokine gene expression in peripheral blood mononuclear cells during graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 1993; 85(3):558-565. [10] SCHMALTZ C, ALPDOGAN O, HORNDASCH KJ, et al. Differential use of Fas ligand and perforin cytotoxic pathways by donor T cells in graft-versus-host disease and graft-versus-leukemia effect. Blood. 2001; 97(9):2886-2895. [11] CHANDRASEKAR B, VEMULA K, SURABHI RM, et al. Activation of intrinsic and extrinsic proapoptotic signaling pathways in interleukin-18-mediated human cardiac endothelial cell death. J Biol Chem. 2004; 279(19):20221-20233. [12] LUO M, YERUVA S, LIU Y, et al. IL-1beta-Induced Downregulation of the Multifunctional PDZ Adaptor PDZK1 Is Attenuated by ERK Inhibition, RXRalpha, or PPARalpha Stimulation in Enterocytes. Front Physiol. 2017;8:61. [13] SABZEVARY-GHAHFAROKHI M, SOLTANI A, LUZZA F, et al. The protective effects of resveratrol on ulcerative colitis via changing the profile of Nrf2 and IL-1β protein. Mol Biol Rep. 2020;47(9):6941-6947. [14] PARK MJ, MOON SJ, LEE SH, et al. Blocking activator protein 1 activity in donor cells reduces severity of acute graft-versus-host disease through reciprocal regulation of IL-17-producing T cells/regulatory T cells. Biol Blood Marrow Transplant. 2014;20(8):1112-1120. [15] MORIYAMA I, ISHIHARA S, RUMI MA, et al. Decoy oligodeoxynucleotide targeting activator protein-1 (AP-1) attenuates intestinal inflammation in murine experimental colitis. Lab Invest. 2008;88(6):652-663. [16] DING L, NING HM, LI PL, et al. Tumor necrosis factor α in aGVHD patients contributed to the impairment of recipient bone marrow MSC stemness and deficiency of their hematopoiesis-promotion capacity. Stem Cell Res Ther. 2020;11(1):119. [17] LIN Y, HU X, CHENG H, et al. Graft-versus-host disease causes broad suppression of hematopoietic primitive cells and blocks megakaryocyte differentiation in a murine model. Biol Blood Marrow Transplant. 2014; 20(9):1290-1300. [18] DING Q, WANG Y, ZHANG AL, et al. ZEB2 Attenuates LPS-Induced Inflammation by the NF-κB Pathway in HK-2 Cells. Inflammation. 2018; 41(2):722-731. [19] BUSS H, HANDSCHICK K, JURRMANN N, et al. Cyclin-dependent kinase 6 phosphorylates NF-κB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. PLoS One. 2012;7(12): e51847. [20] CHEN L, CHEN Z, BAKER K, et al. The short isoform of the CEACAM1 receptor in intestinal T cells regulates mucosal immunity and homeostasis via Tfh cell induction. Immunity. 2012;37(5):930-946. [21] HORST AK, WEGSCHEID C, SCHAEFERS C, et al. Carcinoembryonic antigen-related cell adhesion molecule 1 controls IL-2-dependent regulatory T-cell induction in immune-mediated hepatitis in mice. Hepatology. 2018;68(1):200-214. [22] IIJIMA H, NEURATH MF, NAGAISHI T, et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004; 199(4):471-482. [23] STENGEL ST, FAZIO A, LIPINSKI S, et al. Activating Transcription Factor 6 Mediates Inflammatory Signals in Intestinal Epithelial Cells Upon Endoplasmic Reticulum Stress. Gastroenterology. 2020;159(4): 1357-1374.e10. [24] XU YJ, CHEN FP, CHEN Y, et al. A Possible Reason to Induce Acute Graft-vs.-Host Disease After Hematopoietic Stem Cell Transplantation: Lack of Sirtuin-1 in CD4+ T Cells. Front Immunol. 2018;9:3078. [25] WAICKMAN AT, POWELL JD. Mammalian target of rapamycin integrates diverse inputs to guide the outcome of antigen recognition in T cells. J Immunol. 2012;188(10):4721-4729. [26] WU LX, LA ROSE J, CHEN L, et al. CD28 regulates the translation of Bcl-xL via the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway. J Immunol. 2005;174(1):180-194. [27] DELGOFFE GM, POLLIZZI KN, WAICKMAN AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011; 12(4):295-303. [28] LEE K, GUDAPATI P, DRAGOVIC S, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743-753. [29] SHAO J, EVERS BM, SHENG H. Roles of phosphatidylinositol 3’-kinase and mammalian target of rapamycin/p70 ribosomal protein S6 kinase in K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res. 2004;64(1):229-235. [30] WANG Q, ZHOU Y, JACKSON LN, et al. Nuclear factor of activated T cells (NFAT) signaling regulates PTEN expression and intestinal cell differentiation. Mol Biol Cell. 2011;22(3):412-420. [31] ZHOU Y, WANG Q, GUO Z, et al. Nuclear factor of activated T-cell c3 inhibition of mammalian target of rapamycin signaling through induction of regulated in development and DNA damage response 1 in human intestinal cells. Mol Biol Cell. 2012;23(15):2963-2972. [32] WANG Q, ZHOU Y, RYCHAHOU P, et al. NFAT5 represses canonical Wnt signaling via inhibition of β-catenin acetylation and participates in regulating intestinal cell differentiation. Cell Death Dis. 2013;4(6):e671. [33] ZHANG Y, SANDY AR, WANG J, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117(1):299-308. [34] TRAN IT, SANDY AR, CARULLI AJ, et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123(4):1590-1604. [35] LUO X, XU L, LI Y, et al. Notch pathway plays a novel and critical role in regulating responses of T and antigen-presenting cells in aGVHD. Cell Biol Toxicol. 2017;33(2):169-181. [36] KOBIA FM, PREUSSE K, DAI Q, et al. Notch dimerization and gene dosage are important for normal heart development, intestinal stem cell maintenance, and splenic marginal zone B-cell homeostasis during mite infestation. PLoS Biol. 2020;18(10):e3000850. |
| [1] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [2] | Huang Tao, Jia Zhiqiang, Zhao Xiaoguang, Wang Lei, Fang Liping, Zhai Wenjing, Zhai Shafei, Zhou Yongxin. LncRNA-HOTAIR can regulate differentiation of adipose derived mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 3951-3955. |
| [3] | Qian Xiaofen, Zeng Ping, Liu Jinfu, Chen Cai, Fan Siqi, Nong Jiao, Deng Wei. Comprehensive analysis of competing endogenous RNA network and potential biomarkers in alcohol-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3670-3675. |
| [4] | Yang Shuang, Yan Jinglong. Effects of SOX9 on chondrocyte differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(14): 2279-2284. |
| [5] | Liang Xuezhen, Xie Guoxin, Li Jiacheng, Wen Mingtao, Xu Bo, Li Gang. Identification and analysis of potential key genes in osteonecrosis of the femoral head based on miRNA-mRNA regulatory network [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(11): 1720-1727. |
| [6] | Huang Yangjun, Zhou Honghai, Chen Longhao, Li Jilin, Lu Qingwang, Li Dongyang, He Xinyu. Regulatory role and mechanism of non-coding RNA on steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(11): 1765-1771. |
| [7] | Liu Xin, Yan Feihua, Hong Kunhao. Delaying cartilage degeneration by regulating the expression of aquaporins in rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 668-673. |
| [8] | Xu Huimin, Zhang Suping, Cao Weijie, Li Li, Zhang Ran, Wang Yiran, Wan Dingming. Human umbilical cord blood-derived mesenchymal stem cells in the treatment of refractory acute graft-versus-host disease: a single-arm clinical study [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4921-4927. |
| [9] | Cheng Ning, Zhang Zhizhong. Regulatory effects of LncRNA MEG3 on the proliferation and apoptosis of nucleus pulposus cells in rat intervertebral disc [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(26): 4106-4111. |
| [10] | Yuan Changshen, Rong Weiming, Lu Zhixian, Duan Kan, Guo Jinrong, Mei Qijie. Construction of osteosarcoma miRNA-mRNA regulatory network based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(17): 2740-2746. |
| [11] | Ren Chunmei, Liu Yufang, Xu Nuo, Shao Miaomiao, He Jianya, Li Xiaojie. Non-coding RNAs in human dental pulp stem cells: regulations and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 1130-1137. |
| [12] | Yao Shunhan, Wei Huacheng, Qin Jiagang, Liao Liang. Mogroside V stimulates osteoblast proliferation and differentiation by promoting LncRNA TUG1 expression [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(26): 4129-4134. |
| [13] |
Zhong Ping, Cui Xing.
Baicalin protects intestinal mucosal barrier and intervenes acute graft-versus-host disease by regulating autophagy [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 4028-4032. |
| [14] | Luo Xuanxiang, Feng Hu, Jing Li, Pan Bin. Important roles of non-coding RNA in peripheral nerve repair [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(14): 2271-2276. |
| [15] | Ma Faxin, Sha Weihong, Wang Qiyi, Li Jinliang, Lu Quan, Luo Yujun. Inflammation activated bone marrow mesenchymal stem cell conditioned medium repairs radiation-induced acute injury to intestinal epithelial stem cells [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(9): 1324-1329. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||