Chinese Journal of Tissue Engineering Research ›› 2015, Vol. 19 ›› Issue (5): 674-680.doi: 10.3969/j.issn.2095-4344.2015.05.004
Previous Articles Next Articles
Protective effect of pioglitazone in rat models of radiation-induced heart injury
Song Yang, Wu Rong, Zeng Yue-can, Zhang Zhen-yong, Du Hong-mei
- Second Ward of Oncology Department, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China
-
Revised:2014-11-27Online:2015-01-30Published:2015-03-02 -
Contact:Wu Rong, M.D., Professor, Second Ward of Oncology Department, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China -
About author:Song Yang, Master, Physician, Second Ward of Oncology Department, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China -
Supported by:the National Natural Science Foundation of China, No. 81201803
CLC Number:
Cite this article
Song Yang, Wu Rong, Zeng Yue-can, Zhang Zhen-yong, Du Hong-mei. Protective effect of pioglitazone in rat models of radiation-induced heart injury[J]. Chinese Journal of Tissue Engineering Research, 2015, 19(5): 674-680.
share this article
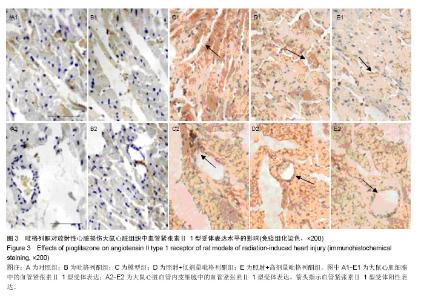
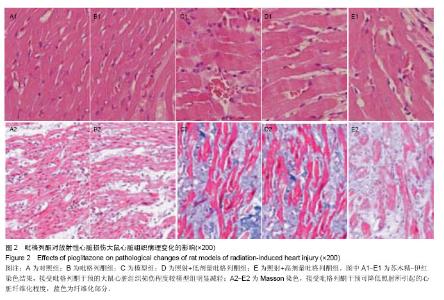
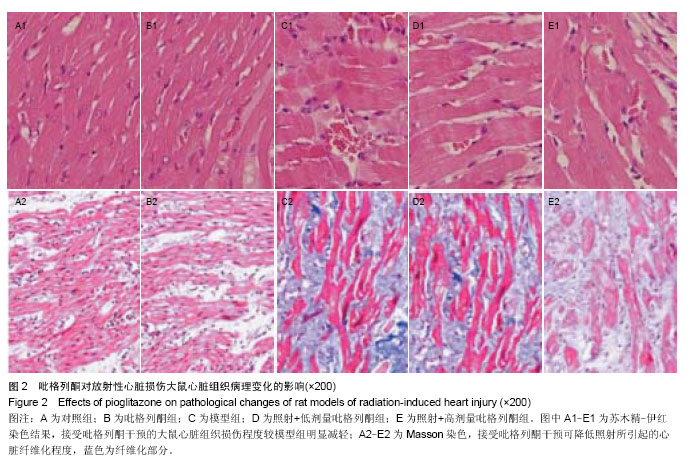
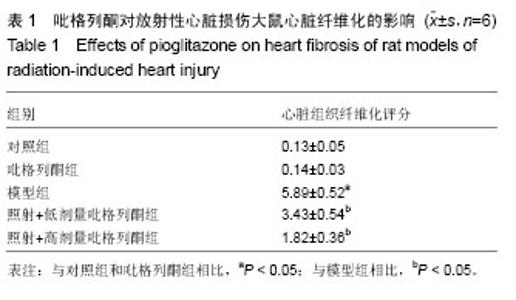
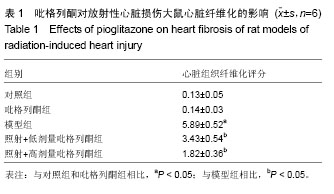
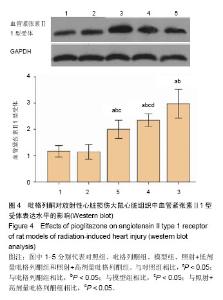
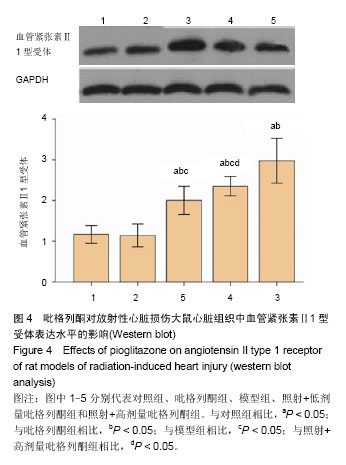
2.3 吡格列酮对放射性心脏损伤大鼠心脏组织中血管紧张素Ⅱ 1型受体表达水平的影响 免疫组化染色结果显示,与对照组及吡格列酮组相比,照射后大鼠心脏组织中血管紧张素Ⅱ 1型受体表达明显增加;而与模型组相比,照射+低剂量吡格列酮组与照射+高剂量吡格列酮组大鼠心脏组织中血管紧张素Ⅱ 1型受体表达减少,提示吡格列酮在放射性心脏损伤中具有保护作用(图3)。 Western blot结果显示,在相对分子质量43 000-50 000附近有明显蛋白表达条带,与对照组及吡格列酮组相比,模型组大鼠心脏组织中血管紧张素Ⅱ 1型受体蛋白表达水平明显增加(P < 0.05),而与模型组相比,照射+低剂量吡格列酮组与照射+高剂量吡格列酮组大鼠心脏组织中血管紧张素Ⅱ 1型受体表达水平减少(P < 0.05),且照射+ 低剂量吡格列酮组大鼠心脏组织中血管紧张素Ⅱ 1型受体蛋白表达水平较照射+高剂量吡格列酮组高(P < 0.05),但2组仍高于对照组及吡格列酮组(P < 0.05),见图4。"

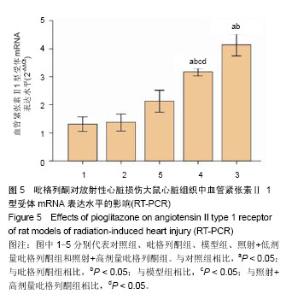
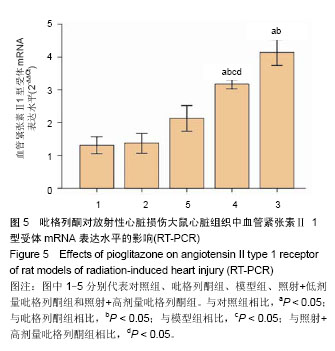
2.4 吡格列酮对放射性心脏损伤大鼠心脏组织中血管紧张素Ⅱ 1型受体mRNA表达水平的影响 实时荧光定量反转录PCR结果显示,与对照组及吡格列酮组相比,模型组大鼠心脏组织中血管紧张素Ⅱ 1型受体mRNA表达水平明显增加(P < 0.05),而与模型组相比,照射+低剂量吡格列酮组与照射+高剂量吡格列酮组大鼠心脏组织中血管紧张素Ⅱ 1型受体mRNA表达水平减少(P < 0.05),且照射+低剂量吡格列酮组大鼠心脏组织中血管紧张素Ⅱ 1型受体mRNA表达水平较照射+高剂量吡格列酮组高(P < 0.05),但2组仍高于对照组及吡格列酮组(P < 0.05;图5)。血管紧张素Ⅱ 1型受体mRNA表达水平变化与蛋白的表达变化一致。"
| [1] Boerma M. Experimental radiation-induced heart disease: past, present, and future. Radiat Res. 2012;178(1):1-6. [2] Boerma M, Hauer-Jensen M. Potential targets for intervention in radiation-induced heart disease. Curr Drug Targets. 2010; 11(11):1405-1412. [3] Filopei J, Frishman W. Radiation-induced heart disease. Cardiol Rev. 2012;20(4):184-188. [4] Lee PJ, Mallik R. Cardiovascular effects of radiation therapy: practical approach to radiation therapy-induced heart disease. Cardiol Rev. 2005;13(2):80-86. [5] Stewart JR, Fajardo LF. Radiation-induced heart disease: an update. Prog Cardiovasc Dis. 1984;27(3):173-194. [6] Andratschke N, Maurer J, Molls M, et al. Late radiation- heart disease after radiotherapy. Clinical importance, radiobiological mechanisms and strategies of prevention. Radiother Oncol. 2011;100(2):160-166. [7] Finch W, Shamsa K, Lee MS. Cardiovascular complications of radiation exposure. Rev Cardiovasc Med. 2014;15(3): 232-244. [8] Lee MS, Finch W, Mahmud E. Cardiovascular complications of radiotherapy. Am J Cardiol. 2013;112(10):1688-1696. [9] Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys. 2002;29(10):2391-2403. [10] Baker JE, Moulder JE, Hopewell JW. Radiation as a risk factor for cardiovascular disease. Antioxid Redox Signal. 2011; 15(7):1945-1956. [11] Balonov M. Third annual Warren K. Sinclair keynote address: retrospective analysis of impacts of the Chernobyl accident. Health Phys. 2007;93(5):383-409. [12] Ermak G, Figge JJ, Kartel NA, et al. Genetic aberrations in Chernobyl-related thyroid cancers: implications for possible future nuclear accidents or nuclear attacks. IUBMB Life. 2003; 55(12):637-641. [13] Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation- associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. 2003;13(3):346-356. [14] Liu HR, Tao L, Gao E, et al. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc Res. 2004; 62(1):135-144. [15] Hart CM, Roman J, Reddy R, et al. PPARgamma: a novel molecular target in lung disease. J Investig Med. 2008;56(2): 515-517. [16] Das SK, Chakrabarti R. Role of PPAR in cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2006;1(2): 193-209. [17] Gao D, Ning N, Hao G, et al. Pioglitazone attenuates vascular fibrosis in spontaneously hypertensive rats. PPAR Res. 2012; 2012:856426. [18] Ehanire T, Ren L, Bond J, et al. Angiotensin II stimulates canonical TGF-β signaling pathway through angiotensin type 1 receptor to induce granulation tissue contraction. J Mol Med (Berl). 2014. in press. [19] Chen K, Chen J, Li D, et al. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone. Hypertension. 2004; 44(5):655-661. [20] Gao S, Wu R, Zeng Y. Up-regulation of peroxisome proliferator-activated receptor gamma in radiation-induced heart injury in rats. Radiat Environ Biophys. 2012;51(1):53-59. [21] Wu R, Zeng Y. Does angiotensin II-aldosterone have a role in radiation-induced heart disease? Med Hypotheses. 2009; 72(3):263-266. [22] 吴荣,马天馨,曾越灿,等.血管紧张素Ⅱ1型受体拮抗剂在大鼠放射性心脏损伤中保护作用的研究[J].中国医科大学学报,2014. in press. [23] Wang J, MacKenzie JD, Ramachandran R, et al. Identifying neutrophils in H&E staining histology tissue images. Med Image Comput Comput Assist Interv. 2014;17(Pt 1):73-80. [24] Pei Y, Fan JJ, Zhang XQ, et al. Repairing the osteochondral defect in goat with the tissue-engineered osteochondral graft preconstructed in a double-chamber stirring bioreactor. Biomed Res Int. 2014;2014:219203. [25] Xia QY, Rao Q, Shen Q, et al. Immunohistochemical study of perivascular epithelioid cell neoplasms. Zhonghua Bing Li Xue Za Zhi. 2013;42(6):381-385. [26] Michalik L, Auwerx J, Berger JP, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58(4):726-741. [27] Macpherson AM, Archer DF, Leslie S, et al. The effect of etonogestrel on VEGF, oestrogen and progesterone receptor immunoreactivity and endothelial cell number in human endometrium. Hum Reprod. 1999;14(12):3080-3087. [28] Horiuchi M, Iwanami J, Mogi M. Regulation of angiotensin II receptors beyond the classical pathway. Clin Sci (Lond). 2012;123(4):193-203. [29] Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645-650. [30] Sime PJ. The antifibrogenic potential of PPARgamma ligands in pulmonary fibrosis. J Investig Med. 2008;56(2):534-538. [31] Genovese T, Cuzzocrea S, Di Paola R, et al. Effect of rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur Respir J. 2005;25(2): 225-234. [32] Iglarz M, Touyz RM, Viel EC, et al. Peroxisome proliferator-activated receptor-alpha and receptor-gamma activators prevent cardiac fibrosis in mineralocorticoid- dependent hypertension. Hypertension. 2003;42(4):737-743. [33] Tsukamoto H, She H, Hazra S, et al. Anti-adipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;21 Suppl 3:S102-105. [34] Fliegner D, Westermann D, Riad A, et al. Up-regulation of PPARgamma in myocardial infarction. Eur J Heart Fail. 2008; 10(1):30-38. [35] Chintalgattu V, Harris GS, Akula SM, et al. PPAR-gamma agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovasc Res. 2007;74(1): 140-150. [36] Zou Y, Komuro I, Yamazaki T, et al. Cell type-specific angiotensin II-evoked signal transduction pathways: critical roles of Gbetagamma subunit, Src family, and Ras in cardiac fibroblasts. Circ Res. 1998;82(3):337-345. [37] Ramires FJ, Sun Y, Weber KT. Myocardial fibrosis associated with aldosterone or angiotensin II administration: attenuation by calcium channel blockade. J Mol Cell Cardiol. 1998;30(3): 475-483. [38] Jawien J, Toton-Zuranska J, Gajda M, et al. Angiotensin-(1-7) receptor Mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. J Physiol Pharmacol. 2012;63(1): 77-85. [39] Ceravolo GS, Montezano AC, Jordão MT, et al. An Interaction of renin-angiotensin and kallikrein-kinin systems contributes to vascular hypertrophy in angiotensin II-induced hypertension: in vivo and in vitro studies. PLoS One. 2014; 9(11): e111117. [40] Lan TH, Huang XQ, Tan HM. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. 2013;22(5):401-407. [41] Zhao SM, Li HW, Guo CY, et al. Cardiac fibrosis in diabetic rats: regulation and mechanism of activation of the PPARgamma signal pathway. Chin J Physiol. 2010;53(4): 262-267. [42] Takeda K, Ichiki T, Tokunou T, et al. Peroxisome proliferator-activated receptor gamma activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102(15):1834-1839. [43] Zhuo J, Moeller I, Jenkins T, et al. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J Hypertens. 1998;16(12 Pt 2):2027-2037. [44] Su EJ, Lombardi DM, Siegal J, et al. Angiotensin II induces vascular smooth muscle cell replication independent of blood pressure. Hypertension. 1998;31(6):1331-1337. [45] Laplante MA, Wu R, El Midaoui A, et al. NAD(P)H oxidase activation by angiotensin II is dependent on p42/44 ERK-MAPK pathway activation in rat's vascular smooth muscle cells. J Hypertens. 2003;21(5):927-936. [46] Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52(4):639-672. |
| [1] | Yang Lijuan, Yao Na, Lu Ruiwen, Liu Xiaoyu, Wang Baojun. Bleomycin combined with angiotensin II for establishing a mouse model of aortic dissection [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(14): 2194-2199. |
| [2] | Liu Ya, Liu Xia, Deng Penghui, Ji Wei, Li Jianping. Exercise effects on myocardial type I, III collagen and angiotensin II/transforming growth factor beta1/Smad2 pathway in diabetic myocardial fibrosis rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4173-4179. |
| [3] | Wen Miaomiao, Fan Junbai. Relationship between biological activities of Apelin and Elabela and diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(35): 5735-5740. |
| [4] | Liu Baoyi1, Li Minde1, Yang Fan2, Li Shaopeng1, Chen Haojie1, Xiao Peng1. Angiotensin II promotes steroid-induced avascular necrosis of the femoral head in mice [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(19): 3044-3049. |
| [5] | Li Qin, Li Yan, Gao Shanshan, Zheng Jintao. Pathways of astragalus polysaccharide inhibiting angiotensin II-induced cardiac hypertrophy [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(19): 3086-3091. |
| [6] | Wang Jing, Han Jiang-hong, Li Qiong. 5-Azacytidine combined with angiotensin II induces differentiation of adipose-derived mesenchymal stem cells into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(29): 4629-4634. |
| [7] | Yan Ping, Hou Jing-ying, Zheng Shao-xin, Long Hui-bao, Zhong Ting-ting, Zhou Chang-qing, Guo Tian-zhu, Wu Quan-hua, Wang Tong. Cardiac stem cells improve the electrophysiological stability and ventricular fibrillation threshold via ANGII/AT1R/TGF-beta1/SMAD/CX43 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2016, 20(28): 4226-4233. |
| [8] | She Shu-song, Zhou Bo, Xu Shun-cai. Effects of different ventilation modes on stress reaction of hypertensive patients treated with hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(35): 5588-5592. |
| [9] | Shi Jing-bao, Xing Wan-hong. Different concentrations of angiotensin II induce directional differentiation of bone marrow mesenchymal stem cells into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2014, 18(50): 8072-8079. |
| [10] | Yao Dan-dan, Ma Rui-xia, Zhai Li-hui, Li Zuo-lin, Li Zhen. Angiotensin II-transient receptor potential channel C6 signaling pathway mediates podocyte injury [J]. Chinese Journal of Tissue Engineering Research, 2014, 18(46): 7447-7451. |
| [11] | Zhu Yuan, Zhang Xiao-dong, Song Jie, Li Hui. Effects of continuous renal replacement therapy on refractory hypertension in maintenance hemodialysis patients [J]. Chinese Journal of Tissue Engineering Research, 2014, 18(18): 2903-2908. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||