Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (34): 5455-5460.doi: 10.3969/j.issn.2095-4344.2014.34.008
Previous Articles Next Articles
Preparation and characterization of ciliary neurotrophic factor sustained-release microcapsules
Li Xiao-li, Wang Ming-bo, Chen Chang-sheng, She Zhen-ding
- Key Laboratory of Biomedical Materials and Implants, Research Institute of Tsinghua University in Shenzhen, Shenzhen 518057, Guangdong Province, China
-
Revised:2014-06-30Online:2014-08-20Published:2014-08-20 -
Contact:She Zhen-ding, M.D., Associate researcher, Key Laboratory of Biomedical Materials and Implants, Research Institute of Tsinghua University in Shenzhen, Shenzhen 518057, Guangdong Province, China -
About author:Li Xiao-li, Master, Engineer, Key Laboratory of Biomedical Materials and Implants, Research Institute of Tsinghua University in Shenzhen, Shenzhen 518057, Guangdong Province, China -
Supported by:the Special Fund by Shenzhen for Biology, Internet and New Energy Resources Development, No. CXB201104250055A
CLC Number:
Cite this article
Li Xiao-li, Wang Ming-bo, Chen Chang-sheng, She Zhen-ding. Preparation and characterization of ciliary neurotrophic factor sustained-release microcapsules[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(34): 5455-5460.
share this article
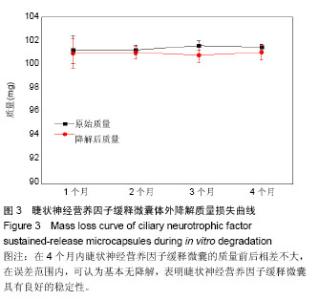
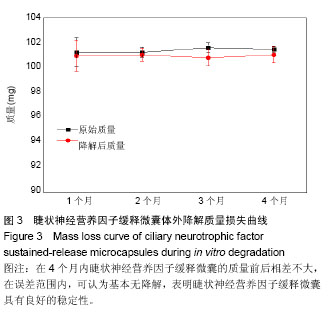
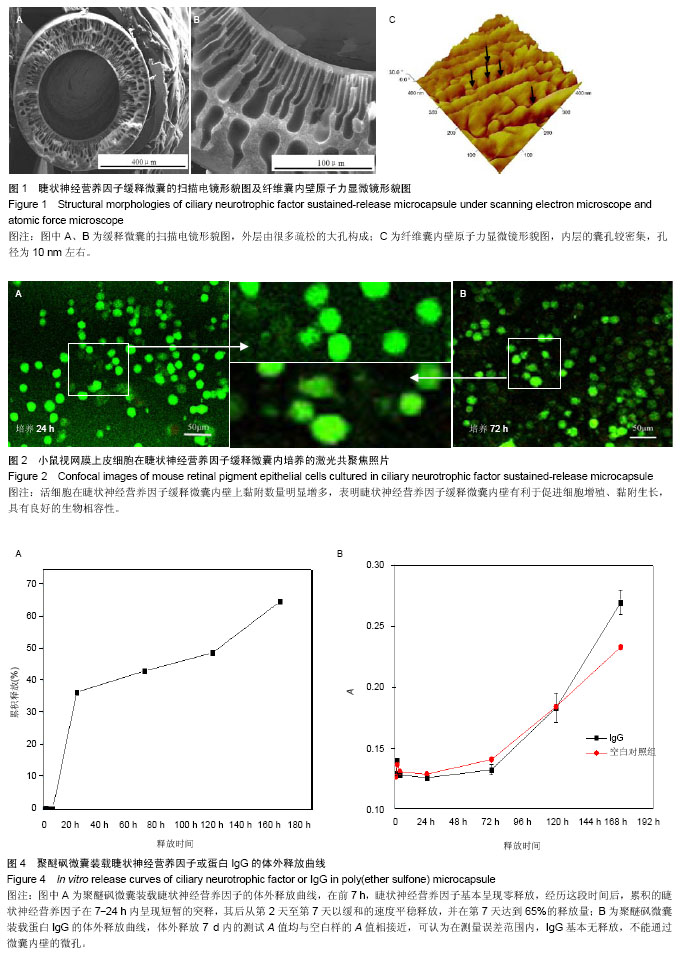
2.1 CNTF缓释微囊微观结构形貌 CNTF缓释微囊的微观形貌如图1所示,外层由很多疏松的大孔构成(图1A,B),内层的囊孔较密集,孔径为10 nm左右(图1C),满足其半渗透性的孔径要求。利用ImageJ软件对10根CNTF缓释微囊100个不同位置的内径及壁厚进行测量,结果显示纤维囊的内径为(398.0±12.6) μm,壁厚(145.0±20.9) μm。本实验制备微囊的直径为Thanos等[33]报道的NT501中聚醚砜中空纤维囊直径的一半,增加了表面积/体积比。 2.2 CNTF缓释微囊的生物相容性评价 阴性对照组的A值为0.634±0.091,CNTF缓释微囊的A值为0.651±0.094,由相对细胞存活率公式得细胞的存活率为102.68%,根据GB/16886标准细胞毒性评价,CNTF缓释微囊的细胞毒性为0级。利用小鼠视网膜上皮细胞黏附在CNTF缓释微囊内壁24,72 h后,对细胞进行Live-Dead染色,由图2可知活细胞在CNTF缓释微囊内壁上黏附数量明显增多,因此,CNTF缓释微囊内壁有利于促进细胞增殖、黏附生长,具有良好的生物相容性。 2.3 CNTF缓释微囊的体外降解性能 为了探究CNTF缓释微囊在眼科植入体内的降解,采用生理盐水为模拟眼液研究了缓释微囊的体外降解性能。由图3降解质量曲线可知,在4个月内CNTF缓释微囊的质量前后相差不大,在误差范围内,可认为基本无降解。因此,CNTF缓释微囊具有良好的稳定性。 2.4 CNTF缓释微囊的体外释放曲线 将一定量CNTF与模拟蛋白IgG分别加载至PES中空纤维膜中,考察它们的在微囊中的体外释放行为。由图4A可知,在前7 h,CNTF基本呈现零释放,这可能是由于CNTF透过内壁的微孔及数百微米的厚壁需要一定的时间所致。经历这段时间后,累积的CNTF在7-24 h内呈现短暂的突释,其后从第2天至第7天以缓和的速度平稳释放,并在第7天达到65%的释放量。而对于模拟蛋白IgG,体外释放7 d内的测试A值均与空白样的A值相接近(图4B),可认为在测量误差范围内,IgG基本无释放,不能通过微囊内壁的微孔。因此,特制结构的聚醚砜微囊对于蛋白的释放具有选择性。"
| [1] Tao W.Application of encapsulated cell technology for retinal degenerative diseases.Expert Opin Biol Ther.2006;6(7): 717-726.
[2] Vervoort R,Wright AF.Mutations of RPGR in X-linked Retinitis Pigmentosa (RP3).Hum Mutat.2002;19:486-500.
[3] 董晓,佘华宁.视网膜色素变性疾病治疗进展及研究现状[J].国际眼科杂志,2011,11(4):633-636.
[4] 艾明,孙明,李岱.视网膜色素变性的治疗进展[J].国际眼科杂志, 2010,10(7):1324-1326.
[5] 蒋沁,曹国凡,胡红莉.视网膜色素变性的研究进展[J].眼视光学杂志,2006,8(2):126-130.
[6] Bustos M,Moreno-Aliaga MJ,Prieto J.Cardiotrophin-1: a new player in energy metabolism with potential therapeutic application. Aging (AlbanyNY).2011;3(8):698-699.
[7] Sale A,Roy Chowdhury SK,Smith DR,et al.Ciliary neurotrophic factor activates NF-κB to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology. 2013;65:65-73.
[8] Sleeman MW,Anderson KD,Lambert PD,et al.The ciliary neurotrophic factor and its receptor, CNTFR alpha.Pharm Acta Helv.2000;4(2-3):265-272.
[9] 李陶,陈意生,王禾.睫状神经营养因子、雪旺氏细胞与周围神经再生[J].国外医学:临床生物化学与检验学分册,2001,22(1): 25-27.
[10] Kang SS,Keasey MP,Arnold SA,et al.Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis.2013;49C:68-78.
[11] Askvig JM,Leiphon LJ,Watt JA.Neuronal activity and axonal sprouting differentially regulate CNTF and CNTF receptor complex in the rat supraoptic nucleus.Exp Neurol. 2012; 233(1):243-252.
[12] Adler R,Landa KB,Manthorpe M,et al.Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979;204(4400): 1434-1436.
[13] Barbin G,Manthorpe M,Varon S.Purification of the Chick Eye Ciliary Neuronotrophic Factor.J Neurochem.1984; 43(5): 1468-1478.
[14] Masiakowski P,Liu HX,Radziejewski C,et al.Recombinant Human and Rat Ciliary Neurotrophic Factors.J Neurochem.1991;57(3):1003-1012.
[15] Lim GJ,Zare S,Van DM,et al.Cell micro-encapsulation.Adv Exp Med Biol.2010;670:126-136.
[16] LaVail MM,Unoki K,Yasumura D,et al.Multiple growth factors, cytokines and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A.1992;89(23):11249-11253.
[17] Roger J,Brajeul V,Thomasseau S,et al.Involvement of Pleiotrophin in CNTF-mediated differentiation of the late retinal progenitor cells.Dev Biol.2006;298:527-539.
[18] LaVail MM,Yasumura D,Matthes MT,et al.Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci.1998;39(3):592-602.
[19] Gaudana, R,Jwala J,Boddu SH,et al.Recent perspectives in ocular drug delivery. Pharm Res.2009;26(5):1197-1216.
[20] Santos E,Orive G,Calvo A,et al.Optimization of 100 μm alginate-poly-L-lysine-alginate capsules for intravitreous administration. J Control Release.2012;158(3):430-450.
[21] Montanucci P,Pennoni I,Pescara T,et al.The functional performance of microencapsulated human pancreatic islet-derived pre-cursor cells. Biomaterials. 2011; 32: 9254-9262.
[22] Rokstada AMA,Lacíkb I,de Vosc P,et al.Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation.Adv Drug Del Rev.2014; 67-68:111-130.
[23] Mendes AC,Baran ET,Reis RL,et al.Fabrication of phospholipid–xanthan microcapsules by combining micro?uidics with self-assembly.Acta Biomaterialia.2013;9(5): 6675-6685.
[24] Wang MB,Feng QL,Niu XF,et al.A spheres-in-sphere structure for improving protein-loading poly(lactide-co-glycolide) microspheres.Polym Degrad Stabil.2010;95:6-13.
[25] Brun-Graeppi AK,Richard C,Bessodes M,et al.Cell microcarriers and microcapsules of stimuli-responsive polymers.J Control Release.2011;149(3): 209-224.
[26] Weiner AL,Gilger BC.Advancements in ocular drug delivery.Vet Ophthalmol.2010;13(6):395-406.
[27] Checa-Casalengua P,Jiang C,Bravo-Osuna I,et al.Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure.J Control Release.2011;156(1): 92-100.
[28] Murua A,Portero A,Orive G,et al.Cell microencapsulation technology: Towards clinical application.J Control Release. 2008;132(2):76-83.
[29] Brun-Graeppi AK,Richard C,Bessodes M,et al.Cell microcarriers and microcapsules of stimuli-responsive polymers. J Control Release.2011;149(3):209-224.
[30] Emerich DF,Orive G,Thanos C,et al.Encapsulated cell therapy for neurodegenerative diseases: From promise to product. Adv Drug Del Rev. 2014;67-68:131-141.
[31] Unger RE,Huang Q,Peters K,et al.Growth of human cells on polyethersulfone (PES) hollow fiber membranes.Biomaterials. 2005;26(14):1877-1884.
[32] 夏泽坤,吕晓龙.聚醚砜中空纤维膜透析性能研究[J].天津工业大学学报, 2007, 26(2):10-13.
[33] Thanos CG,Bell WJ,O'Rourke P,et al.Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng.2004;10(11-12):1617-1622. |
| [1] | Chen Ziyang, Pu Rui, Deng Shuang, Yuan Lingyan. Regulatory effect of exosomes on exercise-mediated insulin resistance diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 4089-4094. |
| [2] | Chen Yang, Huang Denggao, Gao Yuanhui, Wang Shunlan, Cao Hui, Zheng Linlin, He Haowei, Luo Siqin, Xiao Jingchuan, Zhang Yingai, Zhang Shufang. Low-intensity pulsed ultrasound promotes the proliferation and adhesion of human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3949-3955. |
| [3] | Yang Junhui, Luo Jinli, Yuan Xiaoping. Effects of human growth hormone on proliferation and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3956-3961. |
| [4] | Sun Jianwei, Yang Xinming, Zhang Ying. Effect of montelukast combined with bone marrow mesenchymal stem cell transplantation on spinal cord injury in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3962-3969. |
| [5] | Gao Shan, Huang Dongjing, Hong Haiman, Jia Jingqiao, Meng Fei. Comparison on the curative effect of human placenta-derived mesenchymal stem cells and induced islet-like cells in gestational diabetes mellitus rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3981-3987. |
| [6] | Hao Xiaona, Zhang Yingjie, Li Yuyun, Xu Tao. Bone marrow mesenchymal stem cells overexpressing prolyl oligopeptidase on the repair of liver fibrosis in rat models [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3988-3993. |
| [7] | Liu Jianyou, Jia Zhongwei, Niu Jiawei, Cao Xinjie, Zhang Dong, Wei Jie. A new method for measuring the anteversion angle of the femoral neck by constructing the three-dimensional digital model of the femur [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3779-3783. |
| [8] | Meng Lingjie, Qian Hui, Sheng Xiaolei, Lu Jianfeng, Huang Jianping, Qi Liangang, Liu Zongbao. Application of three-dimensional printing technology combined with bone cement in minimally invasive treatment of the collapsed Sanders III type of calcaneal fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3784-3789. |
| [9] | Qian Xuankun, Huang Hefei, Wu Chengcong, Liu Keting, Ou Hua, Zhang Jinpeng, Ren Jing, Wan Jianshan. Computer-assisted navigation combined with minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3790-3795. |
| [10] | Hu Jing, Xiang Yang, Ye Chuan, Han Ziji. Three-dimensional printing assisted screw placement and freehand pedicle screw fixation in the treatment of thoracolumbar fractures: 1-year follow-up [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3804-3809. |
| [11] | Shu Qihang, Liao Yijia, Xue Jingbo, Yan Yiguo, Wang Cheng. Three-dimensional finite element analysis of a new three-dimensional printed porous fusion cage for cervical vertebra [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3810-3815. |
| [12] | Wang Yihan, Li Yang, Zhang Ling, Zhang Rui, Xu Ruida, Han Xiaofeng, Cheng Guangqi, Wang Weil. Application of three-dimensional visualization technology for digital orthopedics in the reduction and fixation of intertrochanteric fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3816-3820. |
| [13] | Sun Maji, Wang Qiuan, Zhang Xingchen, Guo Chong, Yuan Feng, Guo Kaijin. Development and biomechanical analysis of a new anterior cervical pedicle screw fixation system [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3821-3825. |
| [14] | Lin Wang, Wang Yingying, Guo Weizhong, Yuan Cuihua, Xu Shenggui, Zhang Shenshen, Lin Chengshou. Adopting expanded lateral approach to enhance the mechanical stability and knee function for treating posterolateral column fracture of tibial plateau [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3826-3827. |
| [15] | Zhu Yun, Chen Yu, Qiu Hao, Liu Dun, Jin Guorong, Chen Shimou, Weng Zheng. Finite element analysis for treatment of osteoporotic femoral fracture with far cortical locking screw [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3832-3837. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||