Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (在线): 1-13.
Biomimetic Black Phosphorus Nanosystem Regulates Synovial Macrophage Polarization for Osteoarthritis Treatment
Yu Cenqi, Liu Yang, Yu Jiangfeng, Kang Kang,Deng Yaoge, Xia Xiaowei, Zhang Yijian, Zhu Xuesong
- Department of Orthopedics, First Affiliated Hospital of Soochow University, Institute of Orthopedics of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2025-09-27Revised:2026-01-07Online:2026-01-01Published:2026-02-03
CLC Number:
Cite this article
Yu Cenqi, Liu Yang, Yu Jiangfeng, Kang Kang, Deng Yaoge, Xia Xiaowei, Zhang Yijian, Zhu Xuesong. Biomimetic Black Phosphorus Nanosystem Regulates Synovial Macrophage Polarization for Osteoarthritis Treatment[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-13.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
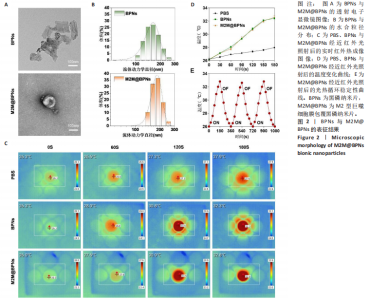
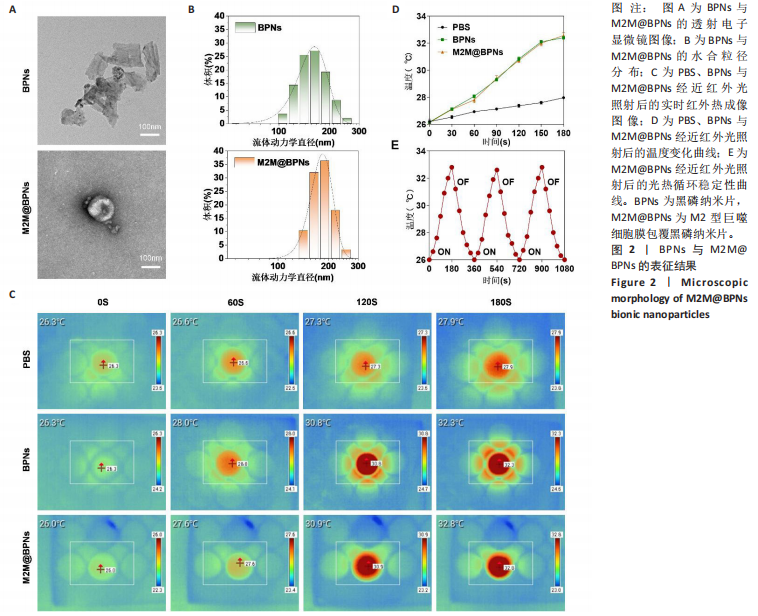
2.1 M2M@BPNs表征结果 透射电子显微镜下可见BPNs与M2M@BPNs呈现超薄纳米片形态,证实了M2M@BPNS表面存在膜结构,见图2A。通过粒径分析确认两种纳米片分散液的粒径范围为100-250 nm,近似呈正态分布,BPNs的平均粒径为(159.37±0.64) nm,M2M@BPNs的平均粒径为(184.76±0.46) nm, M2M@BPNs粒径增加证实了M2型巨噬细胞膜的成功包覆,见图2B。红外热成像结果显示,在808 nm红外光照射下,随着照射时间增加,BPNs与M2M@BPNs分散液的温度逐步升高,在180 s稳定升温6 ℃左右,见图2C,D,结果证实合成的BPNs具有优异光热效应,并且巨噬细胞膜的包覆并未削弱BPNs的光热效应。通过加热-自然冷却循环实验进一步验证了M2M@BPNs的光热稳定性,结果显示该材料具有优异的光热稳定性,见图2E。综上所述,成功制备的仿生纳米片M2M@BPNs表现出优异的光热性能。"
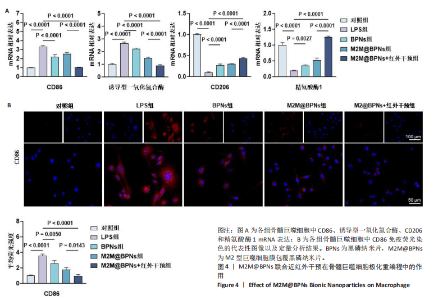
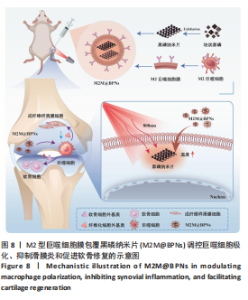
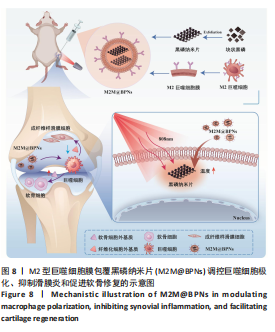
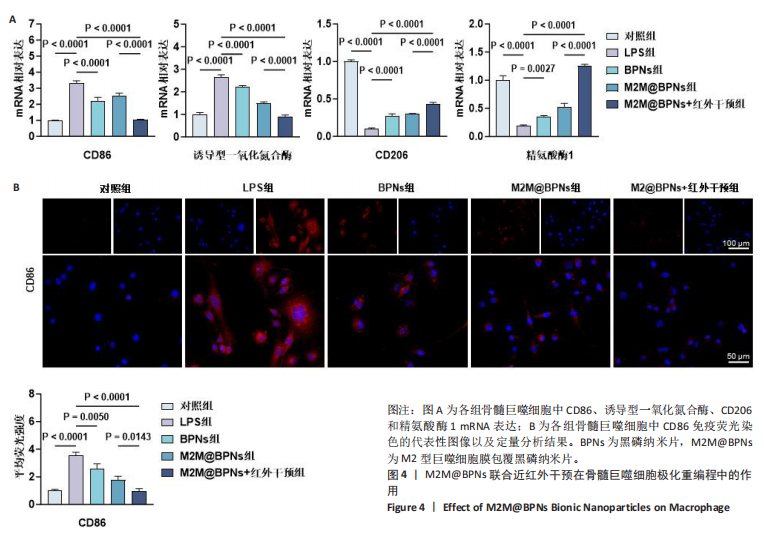
2.3 M2M@BPNs联合近红外干预在巨噬细胞极化重编程中的作用 巨噬细胞极化(M1/M2表型转换)在骨关节炎的滑膜炎症与软骨破坏过程中发挥关键调控作用,巨噬细胞极化失衡可直接驱动关节免疫稳态紊乱并加速疾病进展。RT-qPCR检测结果显示,与对照组相比,脂多糖组CD86和诱导型一氧化氮合酶mRNA表达升高,CD206和精氨酸酶1 mRNA表达下降;与脂多糖组相比,BPNs组、M2M@BPNs组、M2M@BPNs组+红外干预组CD86和诱导型一氧化氮合酶mRNA表达下降,CD206和精氨酸酶1 mRNA表达升高;与BPNs组、M2M@BPNs组相比,M2M@BPNs组+红外干预组CD86和诱导型一氧化氮合酶mRNA表达下降,CD206和精氨酸酶1 mRNA表达升高,见图4A。结果提示,在炎症环境下,M2M@BPNs联合近红外干预能够有效抑制滑膜巨噬细胞向M1表型极化,促进滑膜巨噬细胞向M2表型转化,从而维持关节免疫微环境稳态。免疫荧光染色显示,脂多糖组CD86表达高于对照组,BPNs组、BPNs组、M2M@BPNs组、M2M@BPNs组+红外干预组CD86表达低于脂多糖组,M2M@BPNs组+红外干预组CD86表达低于BPNs组、M2M@BPNs组,见图4B。 综上所述,在炎症条件下,M2M@BPNs联合近红外干预能够显著抑制滑膜巨噬细胞向M1表型极化,恢复关节免疫微环境稳态。"
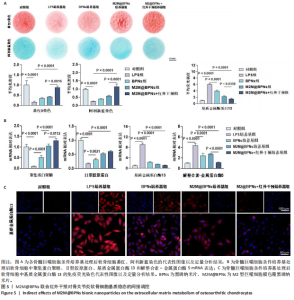
2.4 M2M@BPNs联合近红外干预对骨关节炎软骨细胞基质稳态的间接调控 软骨细胞外基质代谢在骨关节炎的发生发展过程中具有关键作用。此次研究评估M2M@BPNs处理后的骨髓巨噬细胞培养基对骨关节炎软骨细胞基质合成与分解代谢的影响,阿利新蓝与番红染色结果显示,脂多糖培养基组细胞外基质沉积量少于对照组,BPNs培养基组、M2M@BPNs培养基组、M2M@BPNs+红外干预培养基组细胞外基质沉积量多于脂多糖培养基组,M2M@BPNs+红外干预培养基组细胞外基质沉积量多于BPNs培养基组、M2M@BPNs培养基组,见图5A。 RT-qPCR检测结果显示,与对照组相比,脂多糖培养基组中聚集蛋白聚糖和Ⅱ型胶原蛋白mRNA表达降低,基质金属蛋白酶13与解整合素-金属蛋白酶5 mRNA表达升高;与脂多糖培养基组相比,BPNs培养基组、M2M@BPNs培养基组、M2M@BPNs+红外干预培养基组聚集蛋白聚糖和Ⅱ型胶原蛋白mRNA表达升高,基质金属蛋白酶13与解整合素-金属蛋白酶5 mRNA表达降低;与BPNs培养基组、M2M@BPNs培养基组相比,M2M@BPNs+红外干预培养基组聚集蛋白聚糖和Ⅱ型胶原蛋白mRNA表达升高,基质金属蛋白酶13与解整合素-金属蛋白酶5 mRNA表达降低,见图5B。上述结果表明,M2M@BPNs联合近红外干预处理后的骨髓巨噬细胞培养基能够促进基质合成代谢基因的表达、抑制分解代谢相关基因,从而维持软骨细胞外基质稳态。 免疫荧光染色结果显示,脂多糖培养基组基质金属蛋白酶13表达高于对照组,BPNs培养基组、M2M@BPNs培养基组、M2M@BPNs+红外干预培养基组基质金属蛋白酶13表达低于脂多糖组,M2M@BPNs+红外干预培养基组基质金属蛋白酶13表达低于BPNs培养基组、M2M@BPNs培养基组,见图5C。 综上所述,M2M@BPNs联合近红外干预能够有效促进软骨细胞基质合成并抑制降解相关基因和蛋白的表达,从而保护骨关节炎软骨基质稳态。"


2.5 M2M@BPNs联合近红外干预对骨关节炎成纤维细胞代谢稳态的间接调控 成纤维细胞样滑膜细胞的异常活化是骨关节炎发生与进展的重要致病因素之一,因此,此次研究进一步评估了各骨髓巨噬细胞条件培养基对成纤维细胞的代谢功能的影响。 细胞迁移实验结果显示,脂多糖培养基组细胞迁移率高于对照组,M2M@BPNs培养基组、M2M@BPNs+红外干预培养基组细胞迁移率低于脂多糖组,M2M@BPNs+红外干预培养基组细胞迁移率低于BPNs培养基组、M2M@BPNs培养基组,见图6A。结果提示,M2M@BPNs在炎症刺激下能够有效抑制成纤维细胞的异常活化,而联合近红外干预后可能产生相互作用,进一步抑制细胞迁移能力,从而影响骨关节微环境稳态。 RT-qPCR检测结果显示,脂多糖培养基组Ⅰ型胶原蛋白、Ⅱ型胶原蛋白及α-平滑肌肌动蛋白mRNA表达高于对照组,BPNs培养基组、M2M@BPNs培养基组、M2M@BPNs+红外干预培养基组Ⅰ型胶原蛋白、Ⅱ型胶原蛋白及α-平滑肌肌动蛋白mRNA表达低于脂多糖培养基组,M2M@BPNs+红外干预培养基组型胶原蛋白、α-平滑肌肌动蛋白mRNA表达低于BPNs培养基组、M2M@BPNs培养基组,见图6B。 免疫荧光染色结果显示,脂多糖培养基组Ⅰ型胶原蛋白表达高于对照组,BPNs培养基组、M2M@BPNs培养基组、M2M@BPNs+红外干预培养基组Ⅰ型胶原蛋白表达低于脂多糖组,见图6C。 以上结果表明,M2M@BPNs联合近红外干预的骨髓巨噬细胞条件培养基能够显著抑制成纤维细胞的异常活化及相关基因和蛋白的异常表达,从而在炎症环境中维持骨关节微环境稳态。"

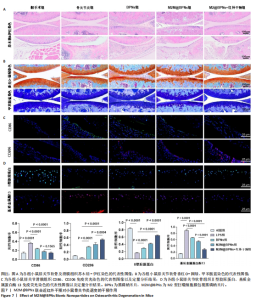
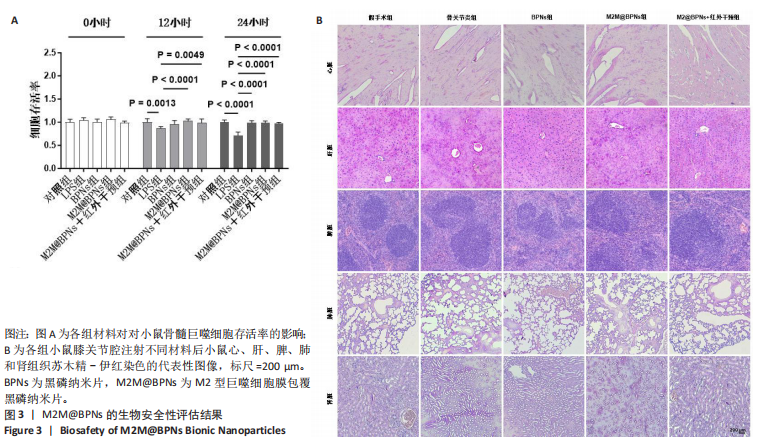
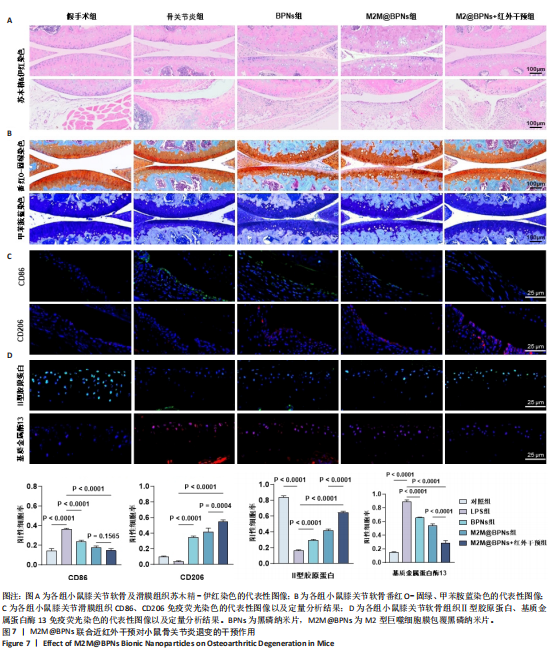
2.6 M2M@BPNs联合近红外干预对小鼠骨关节炎退变的干预作用 2.6.1 实验动物数量分析 30只小鼠全部进入结果分析。 2.6.2 苏木精-伊红染色结果 见图7A。 假手术组关节软骨表面平整,软骨细胞分布均匀、细胞形态与数量正常。骨关节炎组软骨表面粗糙且不连续,浅层可见裂隙或侵蚀至软骨下骨,伴随软骨细胞排列紊乱、数量减少及形态异常,提示软骨明显退变。与骨关节炎组相比,BPNs组与M2M@BPNs组软骨结构有明显改善,软骨表面恢复平整,软骨细胞数量增加并趋于正常排列。M2M@BPNs+红外干预组如昂胡修复效果优于BPNs组、M2M@BPNs组。在滑膜层面,假手术组滑膜衬里细胞呈单层排列,组织结构正常;骨关节炎组表现为衬里细胞层数和细胞密度显著增加;BPNs组与M2M@BPNs组滑膜增生减轻,细胞密度降低;M2M@BPNs+红外干预组滑膜结构至接近假手术组水平。 2.6.3 番红-固绿及甲苯胺蓝染色结果 见图7B。 假手术组软骨基质蛋白聚糖含量丰富,而骨关节炎组蛋白聚糖明显丢失;BPNs组与M2M@BPNs组蛋白聚糖含量明显多于骨关节炎组,而M2M@BPNs+红外干预组对基质蛋白聚糖含量多于BPNs组、M2M@BPNs组。 2.6.4 免疫荧光染色结果 见图7C,D。 在滑膜组织中,与对照组相比,骨关节炎组CD86阳性细胞增加,CD206阳性细胞减少;与骨关节炎组相比,BPNs组、M2M@BPNs组、M2M@BPNs+红外干预组CD86阳性细胞减少,CD206阳性细胞增加;M2M@BPNs+红外干预组CD206阳性细胞多于BPNs组、M2M@BPNs组,表明M2M@BPNs联合近红外干预能够抑制巨噬细胞向M1表型极化、促进巨噬细胞向M2表型转化,从而恢复关节免疫稳态。 在软骨组织中,与对照组相比,骨关节炎组Ⅱ型胶原蛋白阳性细胞比例下降,基质金属蛋白酶13阳性细胞比例升高;与骨关节炎组相比,BPNs组、M2M@BPNs组、M2M@BPNs+红外干预组Ⅱ型胶原蛋白阳性细胞比例升高,基质金属蛋白酶13阳性细胞比例下降;M2M@BPNs+红外干预组Ⅱ型胶原蛋白阳性细胞比例高于BPNs组、M2M@BPNs组,基质金属蛋白酶13阳性细胞比例低于BPNs组、M2M@BPNs组。 上述实验结果表明,M2M@BPNs联合近红外干预通过双向调控细胞外基质代谢(即抑制降解、促进合成)实现对软骨组织的保护作用。"
| [1] KLOPPENBURG M, NAMANE M, CICUTTINI F. Osteoarthritis. Lancet. 2025;405(10472):71-85. [2] ZHANG X, HUANG C, HU Z, et al. Global, regional, and country-specific lifetime risks of osteoarthritis, 1990–2021: a systematic analysis for the global burden of disease study 2021. Glob Health Res Policy. 2025;10(1):29. [3] SAFIRI S, KOLAHI A A, SMITH E, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819-828. [4] ZHAO R, LIANG B, SHI Y, et al. Immunomodulatory biomaterials for osteoarthritis: Targeting inflammation and enhancing cartilage regeneration. Mater Today Bio. 2025;34:102100. [5] THOMSON A, HILKENS MU. Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Front Immunol. 2021;12:678757. [6] GRIFFIN TM, SCANZELLO CR. Innate Inflammation and Synovial Macrophages in Osteoarthritis Pathophysiology. Clin Exp Rheumatol. 2020;120(5):57-63. [7] ZHANG Y, JI Q. Macrophage polarization in osteoarthritis progression: a promising therapeutic target. Front Cell Dev Biol. 2023;11:1269724. [8] YAO Q, WU X, TAO C, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):56. [9] ZHANG Z, WANG R, XUE H, et al. Phototherapy techniques for the management of musculoskeletal disorders: strategies and recent advances. Biomater Res. 2023;27(1):123. [10] XIA S, LIU D, JIANG K, et al. Photothermal driven BMSCs osteogenesis and M2 macrophage polarization on polydopamine-coated Ti3C2 nanosheets/poly(vinylidene fluoride trifluoroethylene) nanocomposite coatings. Mater Today Bio. 2024;27:101156. [11] HSU Y, HE Y, ZHAO X, et al. Photothermal Coating on Zinc Alloy for Controlled Biodegradation and Improved Osseointegration. Adv Sci. 2025;12(9):2409051. [12] XUE Y, ZENG G, CHENG J, et al. Engineered macrophage membrane‐enveloped nanomedicine for ameliorating myocardial infarction in a mouse model. Bioeng Transl Med. 2021;6(2):e10197. [13] DENG W, WANG T, LI L, et al. A review of nanomaterials in osteoarthritis treatment and immune modulation. Regen Biomater. 2025;12:rbaf048. [14] THURAKKAL S, ZHANG X. Recent Advances in Chemical Functionalization of 2D Black Phosphorous Nanosheets. Adv Sci. 2020;7(2):1902359. [15] TONG L, LIAO Q, ZHAO Y, et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1-11. [16] SUN T, LI C, LUAN J, et al. Black phosphorus for bone regeneration: Mechanisms involved and influencing factors. Mater Today Bio. 2024;28:101211. [17] MIAO Y, CHEN Y, LUO J, et al. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact Mater. 2023;21: 97-109. [18] QIU M, TULUFU N, TANG G, et al. Black Phosphorus Accelerates Bone Regeneration Based on Immunoregulation. Adv Sci. 2024;11(1):2304824. [19] LI H, XUE Y, ZHANG T, et al. Black Phosphorus Nanosheets Incorporated Mesenchymal Stem Cell Spheroids for Accelerated Bone Regeneration. ACS Appl Nano Mater. 2025;8(17):8963-8976. [20] QIU S, ZHU F, TONG L. Application of targeted drug delivery by cell membrane-based biomimetic nanoparticles for inflammatory diseases and cancers. Eur J Med Res. 2024;29(1):523. [21] LIU H, SU YY, JIANG XC, et al. Cell membrane-coated nanoparticles: a novel multifunctional biomimetic drug delivery system. Drug Deliv Transl Res. 2023;13(3):716-737. [22] ZHOU K, YANG C, SHI K, et al. Activated macrophage membrane-coated nanoparticles relieve osteoarthritis-induced synovitis and joint damage. Biomaterials. 2023;295:122036. [23] TEO KYW, SEVENCAN C, CHEOW YA, et al. Macrophage Polarization as a Facile Strategy to Enhance Efficacy of Macrophage Membrane‐Coated Nanoparticles in Osteoarthritis. Small Sci. 2022;2(4):2100116. [24] LIAO J, ZHU Z, ZOU J, et al. Macrophage Membrane‐Biomimetic Multi‐Layered Nanoparticles Targeting Synovial Angiogenesis for Osteoarthritis Therapy. Adv Healthc Mater. 2025;14(2): 2401985. [25] WU Y, WAN S, YANG S, et al. Macrophage cell membrane-based nanoparticles: a new promising biomimetic platform for targeted delivery and treatmen. J Nanobiotechnol. 2022;20(1):542. [26] TODA G, YAMAUCHI T, KADOWAKI T, et al. Preparation and culture of bone marrow-derived macrophages from mice for functional analysis. STAR Protoc. 2021;2(1):100246 [27] GOSSET M, BERENBAUM F, THIRION S, et al. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3(8):1253-1260. [28] TAO W, ZHU X, YU X, et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv Mater. 2017;29(1):1603276. [29] LIU Y, HAO R, LV J, et al. Targeted knockdown of PGAM5 in synovial macrophages efficiently alleviates osteoarthritis. Bone Res. 2024;12(1):15. [30] YU T, GAN S, ZHU Q, et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat Commun. 2019;10(1):4353. [31] MERKESTEIN M, LABER S, MCMURRAY F, et al. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat Commun. 2015;6(1):6792. [32] HOU J, WANG H, GE Z, et al. Treating Acute Kidney Injury with Antioxidative Black Phosphorus Nanosheets. Nano Lett. 2020;20(2):1447-1454. [33] GLASSON SS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 Suppl 3:S17-23. [34] HUNTER DJ, BIERMA-ZEINSTRA S. Osteoarthritis. Lancet. 2019;393(10182):1745-1759. [35] SCANZELLO CR, GOLDRING SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012; 51(2):249-257. [36] BONDESON J, WAINWRIGHT SD, LAUDER S, et al. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006; 8(6):R187. [37] SANCHEZ-LOPEZ E, CORAS R, TORRES A, et al. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258-275. [38] ZHANG H, CAI D, BAI X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28(5):555-561. [39] LI B, LIU F, YE J, Et al. Regulation of Macrophage Polarization Through Periodic Photo‐Thermal Treatment to Facilitate Osteogenesis. Small. 2022;18(38):2202691. [40] ZHAO J, LUO Y, ZHANG L, et al. Mild Hyperthermia Accelerates Bone Repair by Dynamically Regulating iNOS/Arg1 Balance in the Early Stage. Adv Sci. 2025;12(8):2409882. [41] YUE H, YUAN L, ZHANG W, et al. Progress on therapeutic applications of polymer decorated black phosphorus and black phosphorus analogues nanomaterials in biomedicine. J Mater Chem B. 2018;6(3):393-400. [42] WANG Z, LIU Z, SU C, et al. Biodegradable Black Phosphorus-based Nanomaterials in Biomedicine: Theranostic Applications. Curr Med Chem. 2019;26(10):1788-1805. [43] ZHANG X, ZHAO M, XIAO X, et al. Inorganic Nanomaterials for Osteoarthritis: From Delivery Vehicles to Therapeutic Agents. ACS Appl Mater Interfaces. 2025;17(28):39845-39862. [44] ZHANG Y, SONG J, LI Y, et al. Diagnostic and Therapeutic Potential of Photo-Responsive Nanomaterials in Osteoarthritis. Aging Dis. 2025. doi: 10.14336/AD.2025.0166. [45] ZENG J, GU C, GENG X, et al. Combined photothermal and sonodynamic therapy using a 2D black phosphorus nanosheets loaded coating for efficient bacterial inhibition and bone-implant integration. Biomaterials. 2023;297:122122. [46] MURRAY PJ, ALLEN JE, BISWAS SK, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41(1):14-20. [47] LOCATI M, CURTALE G, MANTOVANI A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15(1):123-147. [48] WANG H, ZHANG J, LI Z, et al. NIR-programmable 3D-printed shape-memory scaffold with dual-thermal responsiveness for precision bone regeneration and bone tumor management. J Nanobiotechnol. 2025;23(1):300. [49] ZHANG L, CHEN X, CAI P, et al. Reprogramming Mitochondrial Metabolism in Synovial Macrophages of Early Osteoarthritis by a Camouflaged Meta‐Defensome. Adv Mater. 2022;34(30):2202715. [50] GOLDRING MB, GOLDRING SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192(1):230-237. [51] KAPOOR M, MARTEL-PELLETIER J, LAJEUNESSE D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42. [52] LU H, WEI J, LIU K, et al. Radical-Scavenging and Subchondral Bone-Regenerating Nanomedicine for Osteoarthritis Treatment. ACS Nano. 2023;17(6):6131-6146. [53] LOTZ M, MARTEL-PELLETIER J, CHRISTIANSEN C, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72(11):1756-1763. [54] SHEN Y, LIANG L, ZHANG S, et al. A core-shell structure QRu-PLGA-RES-DS NP nanocomposite with photothermal response-induced M2 macrophage polarization for rheumatoid arthritis therapy. Nanoscale. 2018;10(4):1622-1630. [55] YIN W, SUN S, YAO H, et al. Black Phosphorus Nanosheet-Based Composite Biomaterials for the Enhanced Repair of Infectious Bone Defects. ACS Biomater Sci Eng. 2025;11(3):1317-1337. [56] LIAO J, GU Q, LIU Z, et al. Edge advances in nanodrug therapies for osteoarthritis treatment. Front Pharmacol. 2024;15:1402825. [57] MO J, XIE Q, WEI W, et al. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona[. Nature Commun. 2018;9(1): 2480. |
| [1] | Zhang Nan, Meng Qinghua, Bao Chunyu. Characteristics and clinical application of ankle joint finite element models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2343-2349. |
| [2] | Chen Qiuhan, Yang Long, Yuan Daizhu, Wu Zhanyu, Zou Zihao, Ye Chuan. Peri-knee osteotomy for treatment of knee osteoarthritis: optimization of treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2303-2312. |
| [3] | Zhang Zizheng, Luo Wang, Liu Changlu. Application value of finite element analysis on unicompartmental knee arthroplasty for medial knee compartmental osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2313-2322. |
| [4] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [5] | Song Puzhen, Ma Hebin, Chen Hongguang, Zhang Yadong. Effect of bone marrow mesenchymal stem cell-derived exosomes combined with transforming growth factor beta 1 on macrophages [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1616-1623. |
| [6] | Li Linzhen, Jiao Hongzhuo, Chen Weinan, Zhang Mingzhe, Wang Jianlong, Zhang Juntao. Effect of icariin-containing serum on lipopolysaccharide-induced inflammatory damage in human chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1368-1374. |
| [7] | Chen Ju, Zheng Jinchang, Liang Zhen, Huang Chengshuo, Lin Hao, Zeng Li. Effect and mechanism of beta-caryophyllene in mice with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1341-1347. |
| [8] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [9] | Li Hao, Tao Hongcheng, Zeng Ping, Liu Jinfu, Ding Qiang, Niu Chicheng, Huang Kai, Kang Hongyu. Mitogen-activated protein kinase signaling pathway regulates the development of osteoarthritis: guiding targeted therapy with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1476-1485. |
| [10] | You Huijuan, Wu Shuzhen, Rong Rong, Chen Liyuan, Zhao Yuqing, Wang Qinglu, Ou Xiaowei, Yang Fengying. Macrophage autophagy in lung diseases: two-sided effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1516-1526. |
| [11] | Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224. |
| [12] | Zhang Qian, Huang Dongfeng. Weighted gene co-expression network analysis combined with machine learning to screen and validate biomarkers for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1096-1105. |
| [13] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [14] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| [15] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||