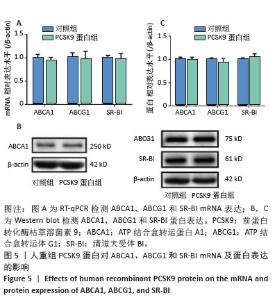
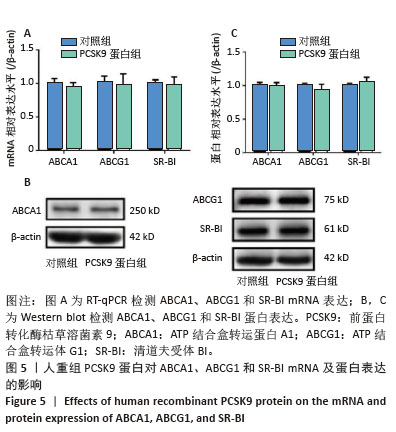
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (13): 3321-3330.doi: 10.12307/2026.623
Previous Articles Next Articles
Impact and mechanism of proprotein convertase subtilisin/kexin type 9 on cholesterol efflux in human monocyte-derived foam cells
Liao Fujun1, 2, Bao Hailong1, 2, Gong Caiwei3, Liu Danan1, 2
- 1Department of Cardiology, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Key Laboratory of Myocardial Remodeling, Department of Cardiology, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 3Zunyi First People’s Hospital, Zunyi 563000, Guizhou Province, China
-
Accepted:2025-07-03Online:2026-05-08Published:2025-12-25 -
Contact:Liu Danan, MD, Chief physician, Department of Cardiology, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Key Laboratory of Myocardial Remodeling, Department of Cardiology, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Liao Fujun, MS, Associate chief physician, Department of Cardiology, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Key Laboratory of Myocardial Remodeling, Department of Cardiology, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:Health Commission Science and Technology Fund Project of Guizhou Province, No. gzwkj2023-300 (to LFJ); Science and Technology Project of Guizhou Province, No. [2021]063 (to LFJ)
CLC Number:
Cite this article
Liao Fujun, Bao Hailong, Gong Caiwei, Liu Danan. Impact and mechanism of proprotein convertase subtilisin/kexin type 9 on cholesterol efflux in human monocyte-derived foam cells[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3321-3330.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
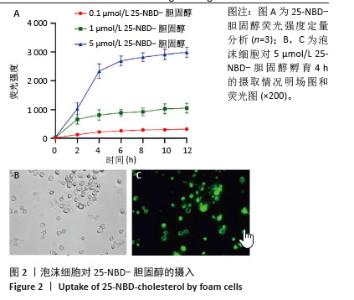
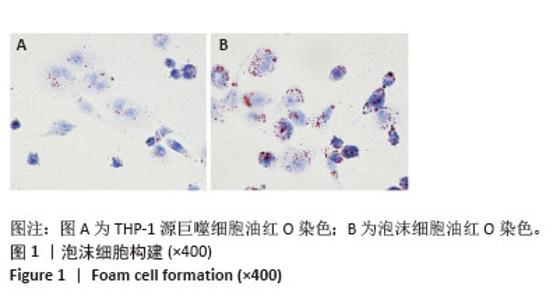
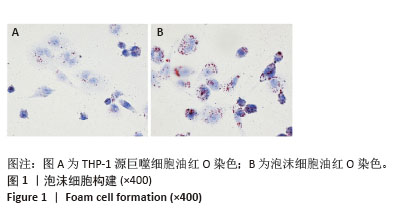
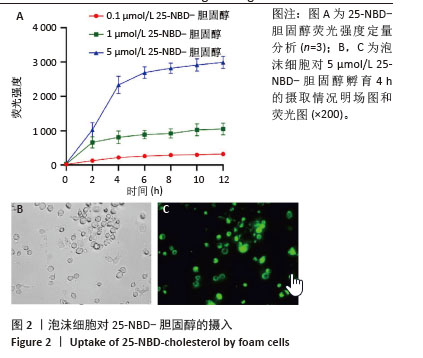
2.2 泡沫细胞对25-NBD-胆固醇的摄入 不同浓度25-NBD-胆固醇孵育液(0.1,1,5 μmol/L)孵育泡沫细胞不同时间(0,2,4,6,8,10,12 h),使用荧光酶标仪检测细胞裂解液的荧光强度,定量分析细胞对不同浓度25-NBD-胆固醇摄入情况(图2A),在相同时间点5 μmol/L组较0.1 μmol/L和1 μmol/L组荧光强度明显升高,泡沫细胞对胆固醇的摄入呈浓度依赖性。25-NBD-胆固醇摄入在孵育4 h时达到平台期。5 μmol/L 25-NBD-胆固醇孵育泡沫细胞4 h荧光强度为2 516.67±117.85,在荧光显微镜下观察荧光标记胆固醇(5 μmol/L 25-NBD-胆固醇)孵育泡沫细胞4 h的摄取情况(图2B和图2C),选择此实验条件用于后续胆固醇流出实验。"
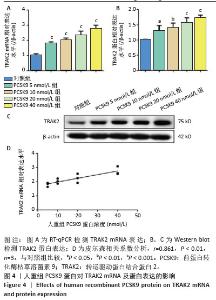
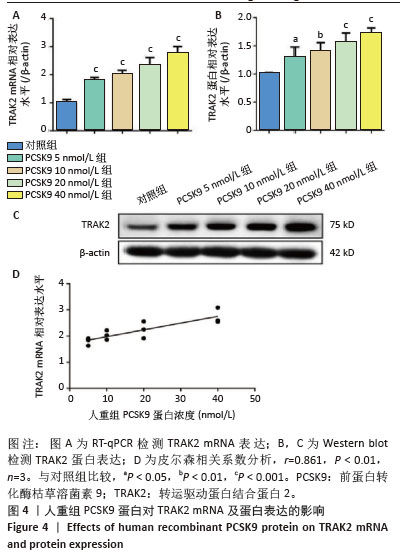
2.4 人重组PCSK9蛋白对TRAK2表达的影响 将不同浓度人重组PCSK9蛋白孵育泡沫细胞24 h,检测TRAK2的mRNA和蛋白表达,结果显示,人重组PCSK9蛋白可增加泡沫细胞中TRAK2的表达,40 nmol/L处理组最显著,相比于对照组,TRAK2 mRNA表达升高(2.73±0.26 vs. 1.01±0.11,图4A,P < 0.001),TRAK2蛋白表达升高(1.71±0.09 vs. 1.00±0.02,图4B,C,P < 0.001)。人重组PCSK9蛋白浓度与TRAK2 mRNA表达呈显著正相关(图4D,Pearson r=0.861,P < 0.01)。因此,在后续实验中人重组PCSK9使用浓度为40 nmol/L。"
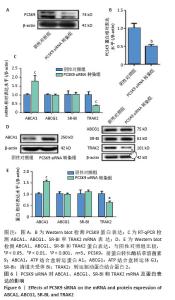
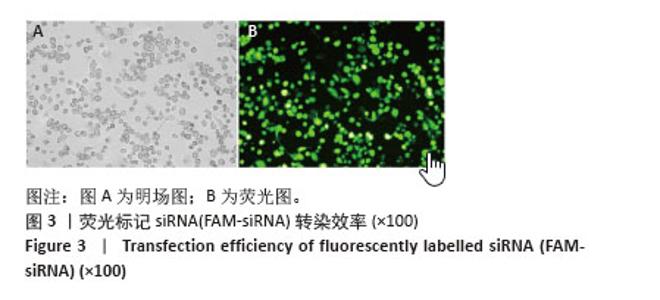
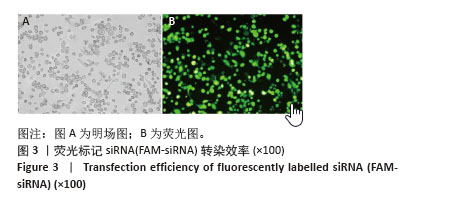
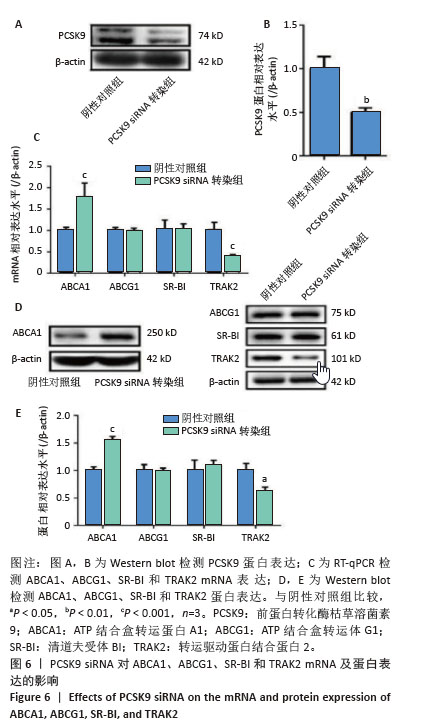
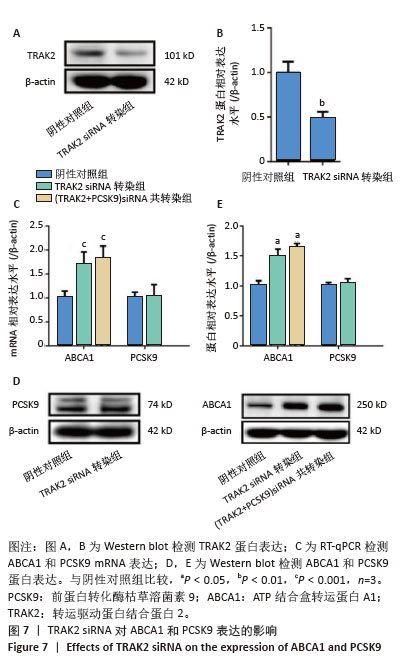
2.6 PCSK9 siRNA对胆固醇转运蛋白表达的影响 用PCSK9 siRNA转染THP-1单核细胞源巨噬细胞,检测PCSK9蛋白表达,结果显示,PCSK9 siRNA转染组的PCSK9蛋白表达低于阴性对照组(非靶向siRNA转染巨噬细胞)(0.50±0.04 vs. 1.00±0.11,图6A,B,P < 0.01)。然后,将巨噬细胞诱导成泡沫细胞,检测TRAK2、ABCA1、ABCG1、SR-BI的表达。PCSK9 siRNA转染组ABCA1 mRNA与蛋白表达高于阴性对照组(非靶向siRNA转染泡沫细胞),分别为1.79±0.30 vs. 1.00±0.07和1.56±0.05 vs. 1.00±0.05,见图6C-E,P均< 0.001,TRAK2 mRNA与蛋白表达低于阴性对照组,分别为0.40±0.03 vs. 1.01±0.17和0.62±0.06 vs. 1.00±0.10,见图6C-E,P < 0.001和P < 0.05,但对ABCG1和SR-BI表达无显著影响,见图6C-E。"
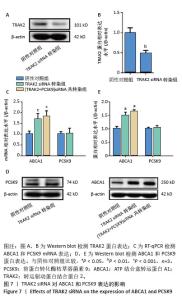
2.7 TRAK2 siRNA对ABCA1和PCSK9表达的影响 用TRAK2 siRNA转染THP-1单核细胞源巨噬细胞,检测TRAK2蛋白表达。TRAK2 siRNA转染组的TRAK2蛋白水平低于阴性对照组(非靶向siRNA转染巨噬细胞)(0.47±0.08 vs. 1.00±0.10,图7A,B,P < 0.01)。然后,将巨噬细胞诱导成泡沫细胞,检测TRAK2 siRNA对泡沫细胞ABCA1和PCSK9表达的影响,结果显示,TRAK2 siRNA转染组ABCA1 mRNA和蛋白表达高于阴性对照组(非靶向siRNA转染泡沫细胞),分别为1.69±0.25 vs. 1.01±0.13和1.49±0.11 vs. 1.00±0.07,见图7C-E,P均< 0.001,但对PCSK9 mRNA和蛋白表达无显著影响,见图7C-E。(PCSK9+TRAK2)siRNA共转染组ABCA1 mRNA和蛋白表达高于阴性对照组,分别为1.80±0.26 vs. 1.01±0.13和1.64±0.05 vs. 1.00±0.07,见图7C-E,P均 < 0.001。(PCSK9+TRAK2) siRNA共转染组与TRAK2 siRNA转染组相比,ABCA1 mRNA和蛋白表达没有显著差异。"
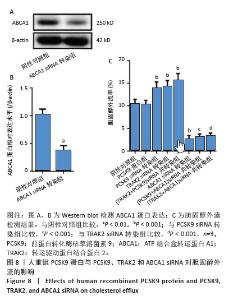
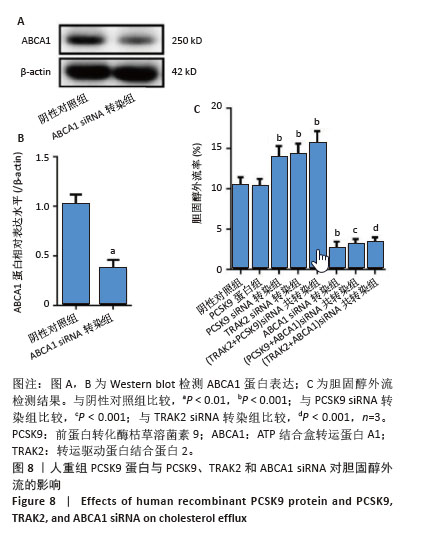
2.8 人重组PCSK9蛋白与PCSK9、TRAK2和ABCA1 siRNA对胆固醇外流的影响 ABCA1 siRNA转染THP-1单核细胞源性巨噬细胞,结果显示,ABCA1 siRNA转染组ABCA1蛋白表达低于阴性对照组(非靶向siRNA转染巨噬细胞)(0.37±0.08 vs. 1.02±0.08,P < 0.01),见图8A,B。胆固醇外流率分析如图8C所示,PCSK9 siRNA转染组、TRAK2 siRNA转染组、(PCSK9+TRAK2) siRNA共转染组胆固醇外流率均高于阴性对照组(非靶向siRNA转染泡沫细胞),分别为(13.80±1.40)%,(14.20±1.31)%,(15.54±1.54)% vs. (10.40±0.97)%,P均< 0.001。ABCA1 siRNA转染组胆固醇外流率低于阴性对照组(2.56±0.86)% vs. (10.40±0.97)%,P < 0.001。(PCSK9+ABCA1) siRNA共转染组胆固醇外流率低于PCSK9 siRNA转染组(3.06±0.70)% vs. (13.80±1.40)%,P < 0.001。(TRAK2+ABCA1) siRNA共转染组胆固醇外流率低于TRAK2 siRNA转染组(3.31±0.66)% vs. (14.20±1.31)%,P < 0.001。人重组PCSK9蛋白对胆固醇外流无明显影响。PCSK9与TRAK2 siRNA共转染相比于PCSK9或TRAK2 siRNA单独转染,胆固醇外流没有显著差异。PCSK9或TRAK2与ABCA1 siRNA共转染相比于ABCA1 siRNA单独转染,胆固醇外流没有显著有差异。 "
| [1] HUMMELGAARD S, VILSTRUP JP, GUSTAFSEN C, et al. Targeting PCSK9 to tackle cardiovascular disease. Pharmacol Ther. 2023; 249:108480. [2] KATZMANN JL, LAUFS U. PCSK9-directed therapies: an update. Curr Opin Lipidol. 2024;35(3):117-125. [3] NICHOLLS SJ. PCSK9 inhibitors and reduction in cardiovascular events: Current evidence and future perspectives. Kardiol Pol. 2023;81(2): 115-122. [4] WANG YM, TAN MY, ZHANG RJ, et al. Acid-Sensing Ion Channel 1/Calpain1 Activation Impedes Macrophage ATP-Binding Cassette Protein A1-Mediated Cholesterol Efflux Induced by Extracellular Acidification. Front Physiol. 2022;12:777386. [5] ESCOLÀ-GIL JC, ROTLLAN N, JULVE J, et al. Reverse Cholesterol Transport Dysfunction Is a Feature of Familial Hypercholesterolemia. Curr Atheroscler Rep. 2021;23(6):29. [6] TAO H, YANCEY PG, BLAKEMORE JL, et al. Macrophage SR-BI modulates autophagy via VPS34 complex and PPARα transcription of Tfeb in atherosclerosis. J Clin Invest. 2021;131(7):e94229. [7] VISHNYAKOVA TG, BOCHAROV AV, BARANOVA IN, et al. SR-BI mediates neutral lipid sorting from LDL to lipid droplets and facilitates their formation. PLoS One. 2020;15(10):e0240659. [8] LIU X, ZHANG Y, LI X, et al. TRAK2 and Its Interacting Partners in the Regulation of Intracellular Trafficking. PLoS ONE. 2023;18(11): e0277354. [9] LI X, ZHANG Y, LIU X, et al. The Role of TRAK Proteins in Neuronal Development and Disease. Front Cell Dev Biol. 2021;9:647019. [10] GANGWAR A, DEODHAR SS, SALDANHA S, et al. Proteomic Determinants of Variation in Cholesterol Efflux: Observations from the Dallas Heart Study. Int J Mol Sci. 2023;24(21):15526. [11] DE MEYER GRY, ZUREK M, PUYLAERT P, et al. Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat Rev Cardiol. 2024;21(5):312-325. [12] CAO D, KHAN Z, LI X, et al. Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism. Cardiovasc Res. 2023;119(9):1825-1841. [13] LIBBY P. The changing landscape of atherosclerosis. Nature. 2021; 592(7855):524-533. [14] MAJDALAWIEH AF, DALIBALTA S, YOUSEF SM. Effects of sesamin on fatty acid and cholesterol metabolism, macrophage cholesterol homeostasis and serum lipid profile: A comprehensive review. Eur J Pharmacol. 2020;885:173417. [15] YVAN-CHARVET L, IVANOV S. Metabolic Reprogramming of Macrophages in Atherosclerosis: Is It All about Cholesterol? J Lipid Atheroscler. 2020;9(2):231-242. [16] ADORNI MP, PAPOTTI B, BORGHI MO, et al. Effect of the JAK/STAT Inhibitor Tofacitinib on Macrophage Cholesterol Metabolism. Int J Mol Sci. 2023;24(16):12571. [17] KONG P, CUI ZY, HUANG XF, et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7(1):131. [18] KLOC M, KUBIAK JZ, GHOBRIAL RM. Macrophage-, Dendritic-, Smooth Muscle-, Endothelium-, and Stem Cells-Derived Foam Cells in Atherosclerosis. Int J Mol Sci. 2022;23(22):14154. [19] GUO H, WEI D, LIU R, et al. A novel therapeutic strategy for atherosclerosis: autophagy-dependent cholesterol efflux. J Physiol Biochem. 2022;78(3):557-572. [20] CHISTIAKOV DA, BOBRYSHEV YV, OREKHOV AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20(1): 17-28. [21] 申燕,王沛,周娟,等.慢性肾功能不全患者血清及硫酸吲哚酚对巨噬细胞脂质聚集的影响[J].南方医科大学学报,2015,35(5):631-638. [22] GUTIERREZ PS. Foam Cells in Atherosclerosis. Arq Bras Cardiol. 2022;119(4):542-543. [23] ZHOU L, LI C, GAO L, et al. High-density lipoprotein synthesis and metabolism (Review). Mol Med Rep. 2015;12(3):4015-4021. [24] HARSCH BA, BORKOWSKI K, WALKER RE, et al. ABCA1 and apoA-I dependent 12-hydroxyeicosatetraenoic acid efflux regulates macrophage inflammatory signaling. bioRxiv [Preprint]. 2024: 2024.07.11.603001. [25] OUIMET M, MARCEL YL. Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arterioscler Thromb Vasc Biol. 2012;32(3):575-581. [26] SOBATI S, SHAKOURI A, EDALATI M, et al. PCSK9: A Key Target for the Treatment of Cardiovascular Disease (CVD). Adv Pharm Bull. 2020;10(4):502-511. [27] MATYAS C, TROJNAR E, ZHAO S, et al. PCSK9, A Promising Novel Target for Age-Related Cardiovascular Dysfunction. JACC Basic Transl Sci. 2023;8(10):1334-1353. [28] WANG J, XIAO Q, WANG L, et al. Role of ABCA1 in Cardiovascular Disease. J Pers Med. 2022;12(6):1010. [29] SEIDAH NG, PRAT A. The Multifaceted Biology of PCSK9. Endocr Rev. 2022;43(3):558-582. [30] LAKE NJ, TAYLOR RL, TRAHAIR H, et al. TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis. Eur Heart J. 2017;38(48):3579-3587. [31] ADORNI MP, CIPOLLARI E, FAVARI E, et al. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis. 2017;256:1-6. [32] ZOU J, XU C, ZHAO ZW, et al. Asprosin inhibits macrophage lipid accumulation and reduces atherosclerotic burden by up-regulating ABCA1 and ABCG1 expression via the p38/Elk-1 pathway. J Transl Med. 2022;20(1):337. [33] IKONEN E, OLKKONEN VM. Intracellular Cholesterol Trafficking. Cold Spring Harb Perspect Biol. 2023;15(8):a041404. [34] JIN P, GAO D, CONG G, et al. Role of PCSK9 in Homocysteine-Accelerated Lipid Accumulation in Macrophages and Atherosclerosis in ApoE-/- Mice. Front Cardiovasc Med. 2021;8:746989. [35] YING Q, RONCA A, CHAN DC, et al. Effect of a PCSK9 inhibitor and a statin on cholesterol efflux capacity: A limitation of current cholesterol-lowering treatments? Eur J Clin Invest. 2022;52(7):e13766. [36] SONG F, LI JZ, WU Y, et al. Ubiquitinated ligation protein NEDD4L participates in MiR-30a-5p attenuated atherosclerosis by regulating macrophage polarization and lipid metabolism. Mol Ther Nucleic Acids. 2021;26:1303-1317. [37] FOGELMAN AM, REDDY ST. Making sense of a seemingly odd connection. Eur Heart J. 2017;38(48):3588-3589. [38] ZHENG S, HUANG H, LI Y, et al. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARγ-LXRα-ABCA1/ABCG1 pathway. Pharmacol Res. 2021;169:105639. [39] OLADOSU O, ESOBI IC, POWELL RR, et al. Dissecting the Impact of Vascular Smooth Muscle Cell ABCA1 versus ABCG1 Expression on Cholesterol Efflux and Macrophage-like Cell Transdifferentiation: The Role of SR-BI. J Cardiovasc Dev Dis. 2023;10(10):416. [40] DERGUNOV AD, BASEROVA VB. Different Pathways of Cellular Cholesterol Efflux. Cell Biochem Biophys. 2022;80(3):471-481. |
| [1] | Song Puzhen, Ma Hebin, Chen Hongguang, Zhang Yadong. Effect of bone marrow mesenchymal stem cell-derived exosomes combined with transforming growth factor beta 1 on macrophages [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1616-1623. |
| [2] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [3] | Pan Hongfei, Zhuang Zhenbing, Xu Baiyun, Yang Zhangyang, Lin Kairui, Zhan Bingqing, Lan Jinghan, Gao Heng, Zhang Nanbo, Lin Jiayu. Inhibitory effects of different concentrations of auranofin on M1 macrophage function and its therapeutic potential in diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1390-1397. |
| [4] | You Huijuan, Wu Shuzhen, Rong Rong, Chen Liyuan, Zhao Yuqing, Wang Qinglu, Ou Xiaowei, Yang Fengying. Macrophage autophagy in lung diseases: two-sided effects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1516-1526. |
| [5] | Fu Zhenyi, Li Junhao, Zhang Yating, He Yunkai, Liu Junyu, Wei Yunhao, Liu Jiaxin. Schwann cells promote peripheral nerve regeneration: retrospect and prospect [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1236-1246. |
| [6] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| [7] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [8] | Zhang Ye, An Zheqing, Xi Xin, Liu Xiaoyan, Hong Wei, Liao Jian. Zoledronic acid-loaded dissolvable microneedle patch inhibits lipopolysaccharide-induced osteoclast differentiation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5115-5124. |
| [9] | Xie Peisen, Guan Zhenpeng, Wei Xianjie, Zhang Keshi, Kang Qingyuan, Xiao Wentao, Guo Xiaoshuai. Cerium dioxide nanoparticles regulate expression of inflammatory factors in M1 macrophages and affect fibroblast co-culture system [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 375-383. |
| [10] | Peng Siwei, Yang Ruifang, Chen Xiaohua, Zheng Zhuoming, Chen Peng, Xiao Yan, Su Youxin, Guo Jiemei. The mechanism by which Zhuanggu Jianxi Decoction inhibits inflammatory response of human synovial macrophages [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4400-4406. |
| [11] | Li Wei, Chai Jinlian, Zhang Bochun, Li Guangzheng, Liu Xiaochen, Wei Chuanfu, Chen Ning, Luo Di, Li Gang, Liang Xuezhen. Preventive effect of lipid-lowering drug targets on the risk of osteonecrosis: genetic information analysis based on the FinnGen and GLGC databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4508-4516. |
| [12] | Tan Yuhang, Li Bo, Tang Minghong, Sun Zeyu, Luo Xu. Isolation, cultivation, identification, and induction of M1/M2 polarization in bone marrow-derived macrophages from C57BL/6 mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3233-3241. |
| [13] | Chen Yuanjun, Lin Sixing, Ji Lichun, Li Dongxiao, Liao Guangzhi, Lin Xingdong. Anti-inflammatory activity and mechanism of Lonicera japonica Thunb.-derived extracellular vesicle-like particles [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3308-3320. |
| [14] | Liu Man, Zhang Kaiwei, Zhu Xu, Ruan Jinghua, Chen Jiunyi, Fei Ji. Mechanism of Shixiang plaster to promote healing of infectious wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2784-2794. |
| [15] | Jiao Taiqiang, Han Xingji, Li Xiangyang, Nan Yi, Yuan Ling, Li Jiaqing, Niu Yang. Mechanism by which Maxing Kugan Decoction intervenes in oleic acid-induced acute lung injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2430-2439. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||