Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (20): 5188-5200.doi: 10.12307/2026.690
Previous Articles Next Articles
Repair of infected bone defect with dual-ion time-sequenced release multifunctional hydrogels
Chen Weifei, Mei Yuandong, Ju Jihui
- Suzhou Ruihua Orthopedic Hospital, Teaching Hospital of Yangzhou University, Suzhou 215000, Jiangsu Province, China
-
Accepted:2025-05-29Online:2026-07-18Published:2025-11-27 -
Contact:Ju Jihui, Chief physician, Associate professor, Suzhou Ruihua Orthopedic Hospital, Teaching Hospital of Yangzhou University, Suzhou 215000, Jiangsu Province, China -
About author:Chen Weifei, MS, Associate chief physician, Suzhou Ruihua Orthopedic Hospital, Teaching Hospital of Yangzhou University, Suzhou 215000, Jiangsu Province, China -
Supported by:Suzhou Science and Technology Plan - Basic Research in Medical Applications, No. SKYD2023026 (to JJH)
CLC Number:
Cite this article
Chen Weifei, Mei Yuandong, Ju Jihui. Repair of infected bone defect with dual-ion time-sequenced release multifunctional hydrogels[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5188-5200.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
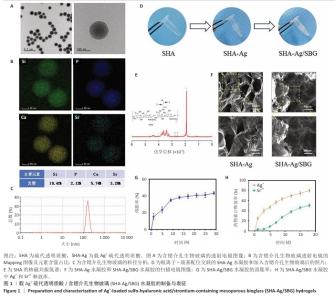
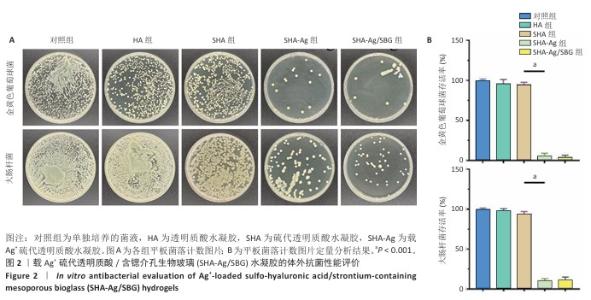
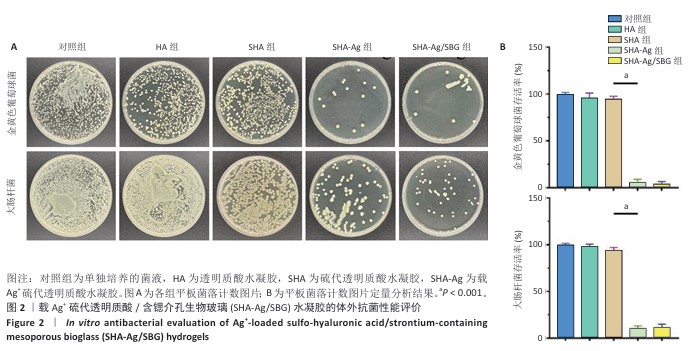
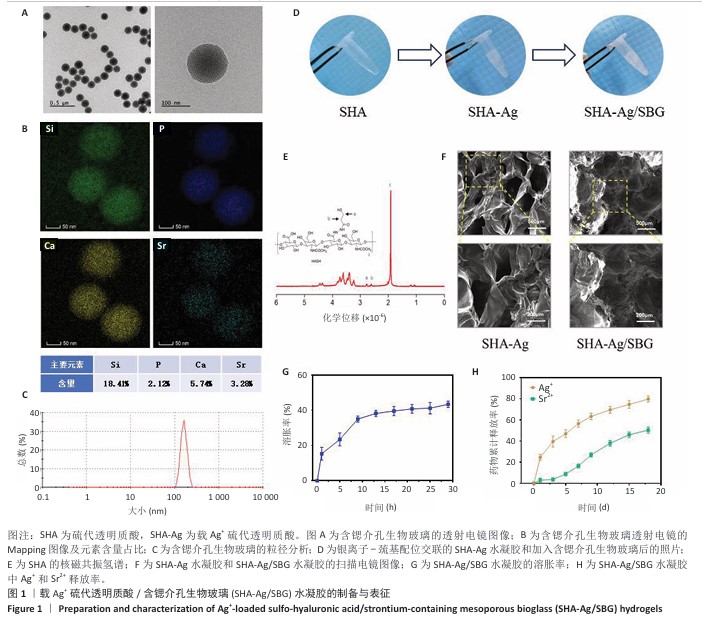
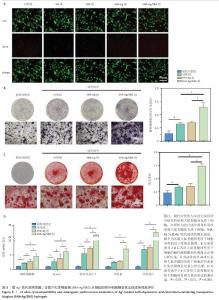
2.1 SHA-Ag/SBG水凝胶的制备与表征分析结果 2.1.1 含锶介孔生物玻璃的合成与表征分析 根据表面模板剂法合成了含锶介孔生物玻璃,透射电镜下可见含锶介孔生物玻璃具有球形结构形态和介孔结构,粒径均一,无明显团聚现象,分散性良好(图1A)。Mapping图谱可见,硅、钙、磷和锶等元素均匀分布在微球内部(图1B),其中Si元素占18.41%,P元素占2.12%,Ca元素占5.74%,Sr元素占3.28%。 动态光散射分析显示含锶介孔生物玻璃粒径均在200 nm左右,有着良好的粒径分布(图1C)。 2.1.2 SHA-Ag与SHA-Ag/SBG水凝胶的合成与表征分析 图1D显示了SHA-Ag/SBG水凝胶的制备过程,向混合均匀的硫代透明质酸溶液中加入Ag?,可见溶液逐渐从液态往固态的转变,加入含锶介孔生物玻璃后溶液由无色透明逐渐向白色转变。图1E显示了硫代透明质酸的核磁共振氢谱,在(2.6-2.8)×10-6处观察到-CH2-CH2-SH的特征峰,证明成功向透明质酸分子结构中引入了巯基(-SH)。图1F分别是 SHA-Ag、SHA-Ag/SBG水凝胶横截面的扫描电镜图像及局部放大图,两组水凝胶均具有多孔结构,显示出均匀互连的三维网状结构,其中SHA-Ag/SBG水凝胶可观察到含锶介孔生物玻璃的存在。 SHA-Ag/SBG水凝胶在10 h达到溶胀平衡后具有明显的体积变化,这有助于水凝胶在感染微环境下吸收渗出物,促进组织愈合(图1G)。图1H显示了SHA-Ag/SBG水凝胶中Sr2+、Ag+释放谱,结果显示:Ag+在早期释放速率快,Sr2+释放较Ag+释放缓慢,第7天,Ag+累计释放率达56.52%,而Sr2+累计释放率仅16.45%。从药物释放曲线可以看出,含锶介孔生物玻璃可以很好地作为离子储载库实现药物缓慢持续释放的目的,这种Ag+早期释放、Sr2+晚期释放能够很好地匹配感染性骨缺损早期抗菌、晚期成骨的需求问题。 "
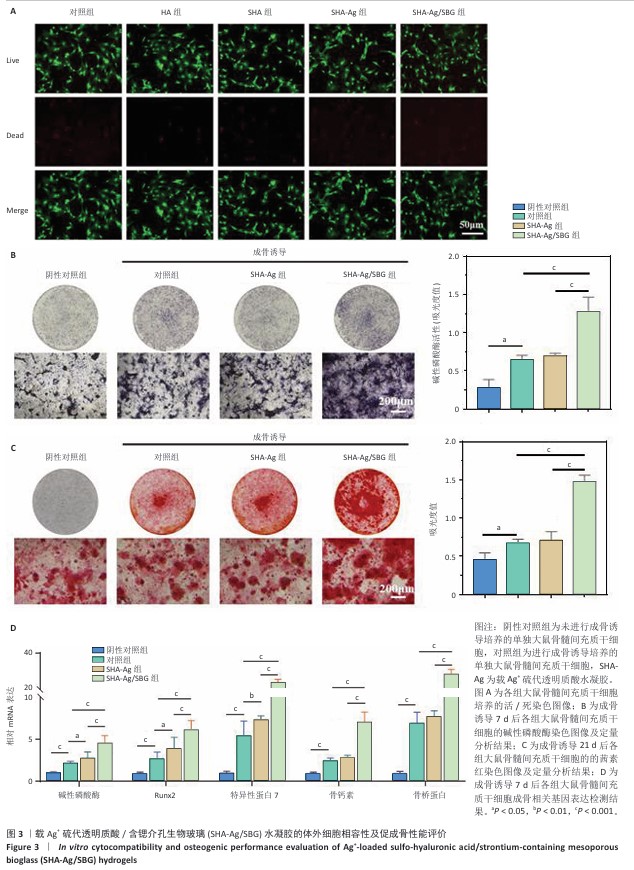
2.3 SHA-Ag/SBG水凝胶的体外细胞相容性及促成骨性能评价结果 2.3.1 活/死染色 如图3A所示,各组均未见明显死细胞,表明各组水凝胶材料对细胞没有明显毒性作用。 2.3.2 成骨特异性碱性磷酸酶染色 碱性磷酸酶染色结果显示,阴性对照组碱性磷酸酶活性低于其他3组,SHA-Ag/SBG组碱性磷酸酶活性高于SHA-Ag组、对照组,差异均有显著性意义,见图3B,表明SHA-Ag/SBG水凝胶促进了大鼠骨髓间充质干细胞的成骨分化。 2.3.3 成骨矿化结节茜素红染色 茜素红染色结果显示,阴性对照组矿化结节形成少于其他3组,SHA-Ag/SBG水凝胶组矿化结节形成多于SHA-Ag水凝胶组、对照组,差异均有显著性意义,见图3C,表明SHA-Ag/SBG水凝胶促进了大鼠骨髓间充质干细胞的成骨分化。 2.3.4 qRT-PCR检测成骨相关基因表达 与阴性对照组比较,对照组、SHA-Ag组碱性磷酸酶、Runx2、特异性蛋白7、骨钙素、骨桥蛋白mRNA表达升高,差异均有显著性意义;与对照组、SHA-Ag组比较,SHA-Ag/SBG组碱性磷酸酶、Runx2、特异性蛋白7、骨钙素、骨桥蛋白mRNA表达升高,差异均有显著性意义,见图3D,表明SHA-Ag/SBG水凝胶促进了成骨相关基因的表达。 "
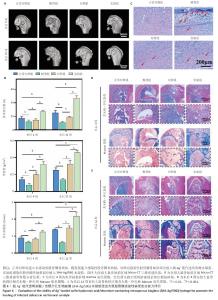
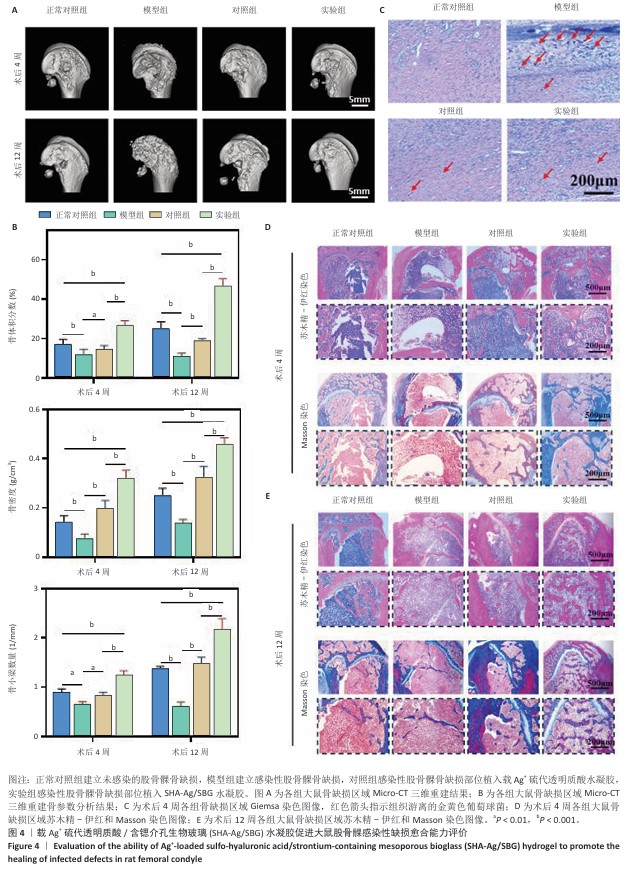
2.4 SHA-Ag/SBG水凝胶促进大鼠股骨髁感染性缺损愈合能力评价结果 2.4.1 实验动物数量分析 40只SD大鼠全部进入结果分析。 2.4.2 Micro-CT检测 模型组术后4,12周股骨髁骨缺损部位骨小梁最为稀疏,仅形成少量新生骨组织;实验组术后4,12周股骨髁骨缺损部位骨小梁最密集,在术后12周时形成了大量新生骨组织(图4A)。定量分析结果显示,与模型组比较,正常对照组、对照组、实验组股骨髁骨缺损部位骨密度、骨体积分数及骨小梁数量均升高,差异均有显著性意义;与正常对照组、对照组比较,实验组股骨髁骨缺损部位骨密度、骨体积分数及骨小梁数量均升高,差异均有显著性意义,见图4B。 2.4.3 Giemsa染色 正常对照组骨缺损区未见细菌染色阳性信号,模型组缺损区可见大量深紫色细菌聚集,提示局部存在显著感染;对照组与实验组均显示细菌数量大幅减少,见图4C。结果表明,引入Ag?的SHA-Ag、SHA-Ag/SBG水凝胶均具有极强的抗菌能力。 2.4.4 苏木精-伊红和Masson染色 苏木精-伊红和Masson染色显示,术后4周,正常对照组骨缺损区可见部分新骨形成,骨小梁数量较少,排列较为松散,同时伴有少量纤维组织填充,整体修复程度有限;模型组骨缺损区部位可见大量炎症细胞浸润,骨组织破坏明显,几乎无新骨形成,胶原沉积稀少;对照组骨缺损区域可见新生骨生成,骨小梁结构不规则,排列较松散,胶原纤维有一定程度沉积;实验组骨缺损区可见大量新骨充填,骨小梁粗大且排列规整,胶原纤维分布丰富且连续,见图4D。术后12周时,各组骨修复进一步进展,正常对照组骨缺损区新骨量略有增加,但仍未完全填充缺损区;模型组骨缺损区仍以纤维组织为主,骨组织修复基本缺失;对照组骨缺损区新生骨面积扩大,但缺损区仍存空隙,骨小梁分布不均;实验组骨缺损区表现出最显著的骨再生效果,缺损区域几乎被致密的新生骨组织完全覆盖,骨小梁增厚并呈连续结构,胶原沉积更加丰富,见图4E。结果表明,SHA-Ag/SBG水凝胶在抑制感染的同时显著促进了新骨形成,对感染性骨缺损具有良好的修复促进作用。 "
| [1] 唐国柯,文根,刘彦斌,等.骨缺损修复生物材料的研究进展[J].中华骨与关节外科杂志,2023,16(2):185-192. [2] MATHIEU L, MOURTIALON R, DURAND M, et al. Masquelet technique in military practice: specificities and future directions for combat-related bone defect reconstruction. Mil Med Res. 2022;9(1):48. [3] MORIARTY TF, METSEMAKERS WJ, MORGENSTERN M, et al. Fracture-related infection. Nat Rev Dis Primers. 2022;8(1):67. [4] WIESE A, PAPE HC. Bone defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthop Clin North Am. 2010;41(1):1-4. [5] GARMANY A, TERZIC A. Global healthspan-lifespan gaps among 183 World Health Organization member states. JAMA Netw Open. 2024;7(12):e2450241. [6] 张宇,尹庆水,张余,等.载银珊瑚羟基磷灰石人工骨的机械性能及修复桡骨大段污染性骨缺损[J]. 中国组织工程研究,2015,19(47): 7567-7574. [7] THABIT AK, FATANI DF, BAMAKHRAMA MS, et al. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81: 128-136. [8] SAINT-PASTOU TERRIER C, GASQUE P. Bone responses in health and infectious diseases: a focus on osteoblasts. J Infect. 2017;75(4):281-292. [9] DONG Q, ZHOU J, FENG M, et al. A review of bacterial and osteoclast differentiation in bone infection. Microb Pathog. 2024;197:107102. [10] TANG R, WU S, JING Y, et al. Application of platelet-rich plasma in traumatic bone infections. Expert Rev Anti Infect Ther. 2021;19(7): 867-875. [11] QIN L, YANG S, ZHAO C, et al. Prospects and challenges for the application of tissue engineering technologies in the treatment of bone infections. Bone Res. 2024;12(1):28. [12] 朱礼威,王江玥,白丁,等.纳米复合甲基丙烯酰明胶水凝胶在不同骨缺损环境中应用的价值[J].中国组织工程研究,2024,28(5): 753-758. [13] CHERNOUSOVA S, EPPLE M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013;52(6): 1636-1653. [14] PARK HJ, KIM JY, KIM J, et al. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009; 43(4):1027-1032. [15] 张骏,尤奇,邹刚,等.锶元素在骨组织工程研究中的应用与进展[J].中国组织工程研究,2019,23(18):2936-2940. [16] CHUANG EY, LIN YC, HUANG YM, et al. Biofunctionalized hydrogel for segmental bone defect repair. Carbohydr Polym. 2024;339:122174. [17] SZEWCZYK A, SKWIRA A, KONOPACKA A, et al. Mesoporous silica-bioglass composite pellets for bone drug delivery system. Int J Mol Sci. 2021;22(9):4708. [18] 王想福,张万乾,郑卉卉,等.感染性骨缺损的治疗进展[J].中国骨与关节杂志,2021,10(6):469-472. [19] OU Z, WEI J, LEI J, et al. Biodegradable Janus sonozyme for infected critical-sized bone defects. Nat Commun. 2024;15(1):10525. [20] ZHANG H, QIAO W, LIU Y, et al. Addressing the challenges of infectious bone defects: a review of recent advances in bifunctional biomaterials. J Nanobiotechnol. 2025;23:257. [21] MASTERS E, RICCIARDI B, BENTLEY K, et al. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol. 2022;20:385-400. [22] WU S, WU B, LIU Y, et al. Mini Review Therapeutic Strategies Targeting for Biofilm and Bone Infections. Front Microbiol. 2022;13:936285. [23] JIAN G, LI D, YING Q, et al. Dual photo‐enhanced interpenetrating network hydrogel for infected bone defect healing. Adv Healthc Mater. 2023;12(25):e2300469. [24] MA L, CHENG Y, FENG X, et al. A Janus-ROS healing system promoting infectious bone regeneration. Adv Mater. 2024;36(2):2307846. [25] HOU S, NIU X, LI L, et al. Injectable hydrogels via nanofibrous protein microparticles for tissue regeneration. Biomaterials. 2019;223:119458. [26] SHAO N, GUO J, GUAN Y, et al. Organic/inorganic composites for bone regeneration. Biomacromolecules. 2018;19(9):3637-3648. [27] SUN X, MA Z, ZHAO X, et al. 3D bioprinting of multicell-laden scaffolds with BMP-4 for bone repair. Bioact Mater. 2021;6(3):757-769. [28] GENG B, LI P, FANG F, et al. Carbon quantum dots for regeneration of infected bone defects. Carbon. 2021;184:375-385. [29] JING X, XU C, SU W, et al. Photosensitive and conductive hydrogel for innerved bone regeneration. Adv Healthc Mater. 2023;12(3):2201349. [30] TURNBULL G, CLARKE J, PICARD F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3(3):278-314. [31] DE PACE R, MOLINARI S, MAZZONI E, et al. Bone Regeneration: A Review of Current Treatment Strategies. J Clin Med. 2025;14(6):1838. [32] 焦振华,刘飞,范文浩,等.胫腓骨感染性骨缺损治疗的研究现状[J].中国矫形外科杂志,2024,32(6):541-546. [33] SAAVEDRA PHV, TRZECIAK AJ, LIPSHUTZ A, et al. Broad-spectrum antibiotics disrupt homeostatic efferocytosis. Nat Metab. 2024;6(9): 1682-1694. [34] ZHAO Q, NI Y, WEI H, et al. Ion incorporation into bone grafting materials. Periodontol 2000. 2024;94(1):213-230. [35] AMENDOLA V, ESTEBAN-GÓMEZ D, FABBRIZZI L, et al. Metal-enhanced H-bond donor tendencies of urea and thiourea. Inorg Chem. 2005;44(24):8690-8698. [36] KHANSA I, SCHOENBRUNNER AR, KRAFT CT, et al. Silver in wound care—friend or foe? A comprehensive review. Plast Reconstr Surg Glob Open. 2019;7(8):e2390. [37] PERCIVAL SL, MCCARTY SM. Silver and alginates: role in wound healing and biofilm control. Adv Wound Care (New Rochelle). 2015;4(7): 407-414. [38] DOHERTY C, BYRNE CV, BAQADER S, et al. Anti-biofilm effects and healing promotion by silver oxynitrate-based dressings. Sci Rep. 2023; 13(1):2014. [39] SHEN Z, MA N, XU J, et al. Metal-ion-controlled hydrogel dressing with enhanced adhesive and antibacterial properties for accelerated wound healing. Mater Today Bio. 2024;26:101039. [40] ALEKSANDRA H, PAULINA K, KAROLINA K, et al. Ag+ complexes as potential therapeutic agents. Curr Med Chem. 2019;26(4):624-647. [41] WANG L, JIANG S, ZHOU J, et al. Strontium-containing biomaterials in regenerative medicine. Bioact Mater. 2025;49:85-120. [42] MARX D, RAHIMNEJAD YAZDI A, PAPINI M, et al. Mechanism of action of strontium in bone. Bone Rep. 2020;12:100273. [43] LODE A, HEISS C, KNAPP G, et al. Strontium-modified calcium phosphate cements for osteoporotic defects. Acta Biomater. 2018;65:475-485. [44] LI J, ZHAO X, XIA Y, et al. Strontium-containing piezoelectric biofilm promotes dentin regeneration. Adv Mater. 2024;36(21):2313419. [45] LIU J, SHI Y, ZHAO Y, et al. Metal–phenolic nanocoating for enhanced osseointegration. Adv Sci. 2024;11(18):2307269. [46] YU Z, WANG Z, CHEN Y, et al. Programmed surface platform for anti-bacterial and bone healing. Biomaterials. 2025;313:122772. [47] YU L, QIAO Y, GE G, et al. Engineered bionic periosteum for bone regeneration. Adv Funct Mater. 2024;34(41):2401109. [48] WANG J, ZHANG Q, WANG H, et al. Sr@Ag-based scaffold against chronic osteomyelitis. Biomaterials. 2025;314:122899. [49] YANG SC, IN Y, ALSHAMMARI SM, et al. Rapid bone destruction caused by multidrug-resistant Pseudomonas aeruginosa septic arthritis: a case report. Medicine (Baltimore). 2024;103(36):e39462. [50] 周子墨,柳达,陈森相,等.3D细胞培养和类器官在骨髓源性间充质干细胞成骨分化中的研究进展[J]. 中国骨质疏松杂志,2022, 28(9):1400-1404. [51] DE WILDT BWM, CUYPERS LAB, CRAMER EEA, et al. The impact of culture variables on a 3D human in vitro bone remodeling model: a design of experiments approach. Adv Healthc Mater. 2023;12(27): e2301205. [52] REMMERS S, MAYER D, MELKE J, et al. Measuring mineralised tissue formation and resorption in a human 3D osteoblast-osteoclast co-culture model. Eur Cell Mater. 2020;40:189-202. [53] BORCIANI G, MONTALBANO G, BALDINI N, et al. Co-culture systems of osteoblasts and osteoclasts: simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020;108:22-45. [54] TOMIZAWA T, NISHITANI K, ITO H, et al. The limitations of mono- and combination antibiotic therapies on immature biofilms in a murine model of implant-associated osteomyelitis. J Orthop Res. 2021;39(2):449-457. [55] MA L, CHENG Y, FENG X, et al. A Janus-ROS healing system promoting infectious bone regeneration via sono-epigenetic modulation. Adv Mater. 2024;36(2):e2307846. [56] WANG L, WU Z, CHEN X, et al. A multifunctional self-assembled hydrogel with bactericidal activity and macrophage metabolic reprogramming for diabetic bone defect repair. Mater Today Bio. 2025;34:102162. |
| [1] | Liu Hongjie, Mu Qiuju, Shen Yuxue, Liang Fei, Zhu Lili. Metal organic framework/carboxymethyl chitosan-oxidized sodium alginate/platelet-rich plasma hydrogel promotes healing of diabetic infected wounds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1929-1939. |
| [2] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [3] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [4] | Zhang Ye, An Zheqing, Xi Xin, Liu Xiaoyan, Hong Wei, Liao Jian. Zoledronic acid-loaded dissolvable microneedle patch inhibits lipopolysaccharide-induced osteoclast differentiation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5115-5124. |
| [5] | Zhou Xiaohui, Wang Siyi, Zhou Qiyun, He Zhao, Jia Yujuan, Wang Yuanbin, Ma Jianwu, Chen Gang, Zheng Feng, Chu Genglei. Nanohydroxyapatite-polyether carbonate urethane electrospinning membrane promotes bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5134-5142. |
| [6] | Diao Youlu, Gao Jia, Pan Guoqing. Recruitable tissue repair biomaterials: advantages of regulating cell and factor migration and improving tissue integration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5270-5281. |
| [7] | Wang Liang, Zhang Xin, He Wei, Wang Jian. Clinical application and prospects of MXene-based materials for the repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5282-5294. |
| [8] | Xu Yawei, Meng Shilong, Zhang Xu, Wang Chengjie, Yuan Yifeng, Shi Xiaolin, Wang Jiao, Liu Kang . Repairing bone defects with active ingredients of traditional Chinese medicine combined with hydrogels: successes and challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5295-5303. |
| [9] | Tang Hao, Zhong Qian, Wu Honghan, Wu Hengpeng, Wu Xingkai, Wa Qingde. 3D-printed biodegradable polyester-based scaffolds in bone regeneration therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5304-5311. |
| [10] | Wang Zitong, Wu Zijian, Yang Aofei, Mao Tian, Fang Nan, Wang Zhigang. Biomaterials regulate microenvironment imbalance for treating spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5321-5330. |
| [11] | Xu Wenhe, Li Xiaobing, Liu Fang. Functionalized biomimetic mineralized collagen modified orthopedic implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 516-527. |
| [12] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [13] | Zhang Qiya, Tong Yixiang, Yang Shijiao, Zhang Yumeng, Deng Ling, Wu Wei, Xie Yao, Liao Jian, Mao Ling. In vitro biocompatibility of graded glass infiltrated ultra-translucent zirconia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 443-450. |
| [14] | Gong Yukang, Ye Gaoqi, Wang Chenhao, Chen Dejin, Gao Wenshan. Effects and mechanisms of natural polyphenol-based hydrogels in promoting bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3675-3686. |
| [15] | Wang Hao, He Qin, Wang Pingxi, Zhang Jun, Wu Zhilin. Deferoxamine-loaded strontium alginate hydrogel promotes the repair of skull injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3609-3617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||