Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 229-237.doi: 10.12307/2025.580
Previous Articles Next Articles
Advances and application of neutrophil extracellular traps and activated platelets in lung cancer research
Yu Daiyao1, 2, Shi Ping2, Yang Lan1, 2, Li Zhishu2, Lu Yongping2
- 1North Sichuan Medical College, Nanchong 637000, Sichuan Province, China; 2Guangyuan Central Hospital, Guangyuan 628017, Sichuan Province, China
-
Received:2024-12-16Accepted:2025-02-20Online:2026-01-08Published:2025-07-02 -
Contact:Shi Ping, MS, Master’s supervisor, Chief physician, Guangyuan Central Hospital, Guangyuan 628017, Sichuan Province, China -
About author:Yu Daiyao, Master candidate, North Sichuan Medical College, Nanchong 637000, Sichuan Province, China; Guangyuan Central Hospital, Guangyuan 628017, Sichuan Province, China -
Supported by:Natural Science Foundation of Sichuan Province, No. 2023NSFSC0329 (to SP)
CLC Number:
Cite this article
Yu Daiyao, Shi Ping, Yang Lan, Li Zhishu, Lu Yongping. Advances and application of neutrophil extracellular traps and activated platelets in lung cancer research[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 229-237.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
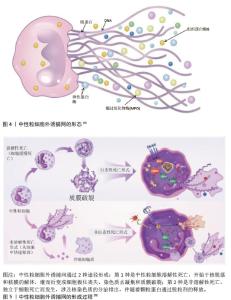
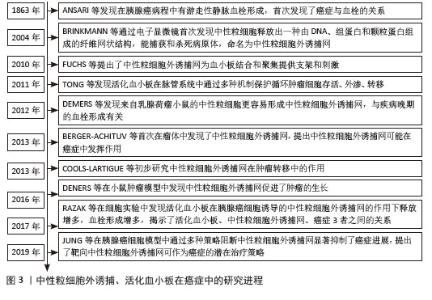
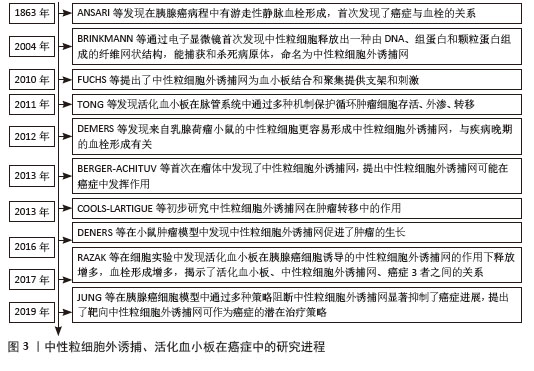
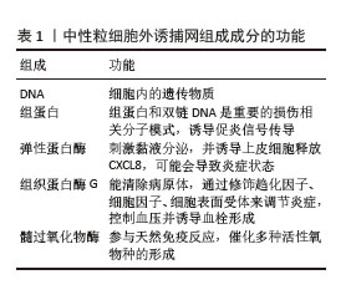
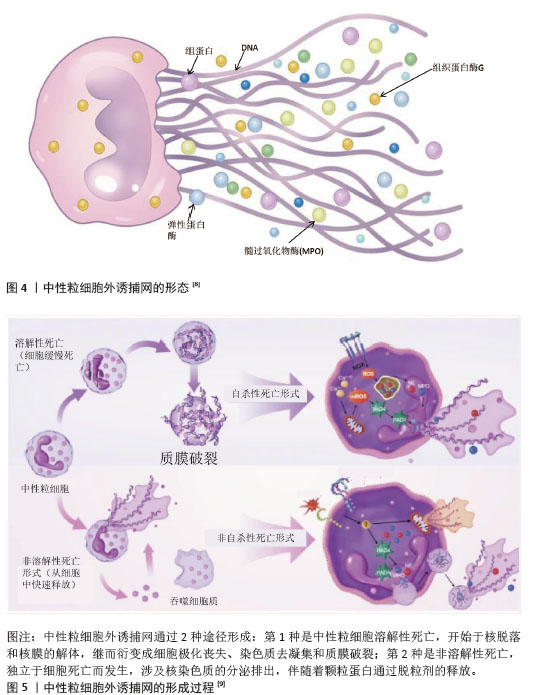
2.2 肺癌与NETs 2.2.1 NETs概念 中性粒细胞是血液中数量最多的白细胞类型,占健康成人白细胞的50%-70%,其作为先天免疫防御的第一道防线,通过吞噬作用、脱颗粒和产生NETs来发挥作用。2004年,BRINKMAN等[5]首次提出了中性粒细胞外捕获网这个概念,即中性粒细胞在各种刺激下从细胞内释放到细胞外的一种以DNA为骨架、表面镶嵌多种蛋白的纤维网状结构,见图4。蛋白质分子包括中性粒细胞弹性蛋白酶、组织蛋白酶G、髓过氧化物酶和组蛋白,见表1。NETs主要通过中性粒细胞的溶解性死亡分泌,这种细胞死亡方式是一种炎性细胞死亡,不同于普通的细胞凋亡和坏死,其特征是多形核细胞的形态学变化,包括染色质解聚、分叶核形态丧失、核膜破坏以及随后的核和细胞质内容物的混合,最后质膜破裂将胞内内容物释放到细胞外环境[6],见图5。最初,NETs被发现作为一种感染防御机制,负责捕获和杀死细胞外病原体。后来,随着对NETs的深入研究,发现NETs在非感染性疾病中也发挥着重要作用,如糖尿病、动脉粥样硬化、自身免疫性疾病、血栓形成、癌症等[7]。NETs与癌症之间的关联是近年来的研究热点。"
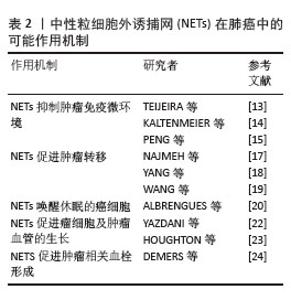
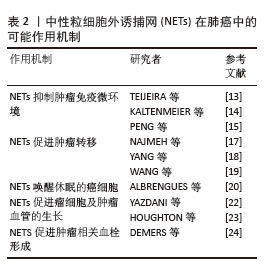
2.2.2 NETs在肺癌中的可能作用机制 (1)肺癌促进NETs形成:肿瘤细胞本身可以在没有任何其他刺激的情况下诱导NETs的产生。在肿瘤微环境中,癌细胞可以释放多种细胞因子,如白细胞介素8、高迁移率组蛋白B1、粒细胞集落刺激因子、趋化因子配体1及趋化因子配体2等,从而诱导中性粒细胞释放NETs。此外,肿瘤细胞分泌的组织蛋白酶C激活中性粒细胞膜结合蛋白酶3、白细胞介素1β和核因子κB,从而上调白细胞介素6和CCL3表达,组织蛋白酶C-中性粒细胞膜结合蛋白酶3-白细胞介素1β轴诱导了NETs的形成[10]。LI等[11]发现在肺癌小鼠模型中肺癌细胞释放的细胞外RNA促进炎症细胞因子白细胞介素1β的表达,降低肺上皮细胞中血管细胞黏附分子1的表达,进而激活上皮细胞间接诱导NETs形成。ZHOU等[12]发现小鼠肺癌细胞主动释放高迁移率组蛋白1,激活HMGB1-TLR4-Myd88-p38/ERK信号通路来诱导NETs的形成。此外,癌症相关成纤维细胞衍生的淀粉样蛋白β也能与中性粒细胞表面的CD11b受体作用以诱导NETs形成。 (2) NETs促进肺癌的发展:NETs形成后通过各种机制促进肺癌进展。首先,在NETs的帮助下,肿瘤细胞可以逃避细胞毒性免疫攻击。NETs作为一个网状纤维结构,能捕获肿瘤细胞并在其表面形成物理屏障,进而保护肿瘤免受NK细胞和T细胞毒性免疫攻击[13]。NETs还可能通过PD-1/PD-L1轴直接引起T细胞的耗竭和功能失调,从而允许肿瘤细胞免疫逃逸[14]。NETs除了物理保护肿瘤细胞免受NK细胞的攻击外,还存在损害NK细胞抗肿瘤功能的其他机制。例如,NETs通过增加转化生长因子β的分泌来激活血小板以损害NK细胞介导的抗肿瘤功能[15]。其次,NETs促进肿瘤的转移[16]。NAJMEH等[17]通过构建小鼠肺癌模型模拟术后炎症环境,发现在炎症背景下NETs上的β1-整合素表达上调,这增加了NETs对体内癌细胞的黏附,并且当使用脱氧核糖核酸(DNase)酶或阻断β1-整合素时,癌细胞对NETs的黏附减弱,肺癌细胞转移也减少。癌症转移与NETs的DNA成分有关,YANG等[18]发现跨膜蛋白CCDC25作为癌细胞上的NET-DNA受体,感受细胞外DNA并随后激活ILK-β-parvin通路以增强细胞运动迁移,促进癌症转移,而且当敲除CCDC25后,NETs介导的癌细胞转移也消除。WANG等[19]研究发现在非小细胞肺癌细胞系(A549和SK-MES-1)和肺癌患者组织中NETs表达均增加,长链非编码RNA MIR503HG表达下调。MIR503HG低表达能激活NF-κB/NOD样通路来诱导NLRP3炎性小体的激活,促进上皮-间充质转化,从而导致癌细胞转移和侵袭。在小鼠模型中,ALBRENGUES等[20]发现在烟草烟雾暴露或鼻腔滴注脂多糖引起的持续性炎症背景下NETs生成,其释放的中性粒细胞弹性蛋白酶和基质金属蛋白酶9依次裂解细胞外基质中的层粘连蛋白,经蛋白水解重塑的层粘连蛋白结合在休眠癌细胞上表达整合素α3β1,激活FAK-ERK-MLCK-YAP信号级联反应,从而唤醒癌细胞的觉醒,驱动小鼠肺部的癌症复发。最后,NETs促进肿瘤的生长[21]。NETs释放中性粒细胞弹性蛋白酶激活癌细胞上的Toll样受体4,导致过氧化物酶体增殖物激活受体γ共激活因子1α上调,诱导线粒体代谢增强,为加速肿瘤生长提供能量[22]。 中性粒细胞弹性蛋白酶也能降解胰岛素受体底物1,进一步激活磷脂酰肌醇3激酶,加强其与血小板衍生生长因子受体之间的相互作用,促进肿瘤的生长[23]。此外,NETs中的基质金属蛋白酶9、组织蛋白酶G可以诱导血管内皮生长因子的表达,促进肿瘤的生长和血管生成。在肺癌小鼠模型中,DEMERS等[24]观察到中性粒细胞增加和NETs形成增强,并通过免疫染色发现小鼠肺静脉中含有富含血管性血友病因子和纤维蛋白的血栓,而对照小鼠没有观察到血栓的迹象,这表明癌细胞诱导的NETs导致血栓形成。总之,肺癌细胞诱导NETs形成,NETs反过来通过多种作用机制促进肺癌的生长、进展、转移,见表2,给患者造成不良的预后。 "
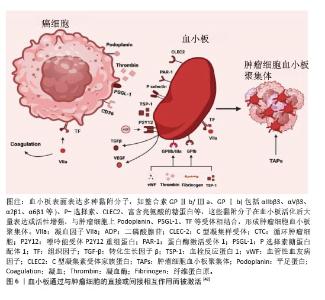
2.2.3 NETs作为肺癌的新型标志物 研究表明,肺癌患者血浆中NETs水平显著升高,有望作为重要的生物标志物[25]。RAYES等[26]通过ELISA法检测60例初治肺癌患者及15名健康个体血浆中的循环NETs水平,与健康对照组相比,肺癌患者的NETs水平明显升高,并且随着肿瘤的分期而升高,表明NETs水平是晚期癌症的强预后因素。基于NETs相关基因的表达模式,ZUO等[27]通过一致性聚类分析得到了肺腺癌的2个NETs相关簇。高NETs评分聚类与良好的总生存期、丰富的免疫细胞浸润、高免疫评分、高SROMA评分和高估计评分以及免疫应答信号通路的高活性相关,低风险的肺腺癌患者具有显著良好的总生存期,NETs相关风险评分和临床分期可作为肺腺癌患者的独立预后因素。基于LASSO算法构建预后特征,筛选出6个具有显著预后价值的NETs相关基因(G0S2、KCNJ15、S100A12、AKT2、CTSG和HMGB1)。 而FANG等[28]对非小细胞肺癌中NETs相关的长链非编码RNA进行全方位生物信息学分析,通过利用单变量Cox回归筛选出19个与总生存期相关的长链非编码RNA,再利用Lasso回归和多因素Cox回归筛选出12个与非小细胞肺癌患者预后明显相关的长链非编码RNA。在另一项临床试验中发现肺癌患者外周血中citH3、cfDNA明显高于健康对照组,并且cfDNA水平与临床分期相关,而早期和晚期肺癌患者citH3水平却没有显著差异,通过受试者工作特征曲线证明citH3、cfDNA、citH3+cfDNA对肺癌的诊断能力依次升高[29]。NETs在肺癌中的表达增加,并且与分期有关,可以被确认为肺癌诊断和监测预后的生物标志物,但有时候也会受到其他多种因素的影响,NETs可能缺乏特异性。 2.3 活化血小板与肺癌 血小板由骨髓巨核细胞产生并释放进入外周血而形成。正常静息状态下,血小板表面光滑,呈双凸碟形,直径为2-5 μm。传统上,血小板的主要功能为止血和血栓形成。但是,随着对血小板越来越多的研究,发现血小板与癌症的关系密切,其中活化血小板在癌症发生发展过程中扮演着重要角色。早在1865年,法国教授ARMAND TROUSSEAU就已报道了癌症与静脉血栓形成之间的联系,表明癌症发展与血小板活性和血凝块形成之间存在相互作用[30]。此后,越来越多的研究表明活化血小板可以参与肿瘤发生的所有阶段,既可以作为保护肿瘤细胞的“防御者”,又可以作为促进癌症进展的积极“参与者”。 2.3.1 肺肿瘤细胞诱导血小板活化 在肺癌中,肿瘤细胞可以通过多种机制诱导血小板活化。肿瘤细胞可释放多种可溶性递质来激活血小板,如腺苷二磷酸、凝血酶、组织蛋白酶B、基质金属蛋白酶或血栓素A2激活血小板。鬼臼素是一种Ⅰ型跨膜唾液糖蛋白,在肺癌中高表达,通过与血小板表面C型凝集素样受体2作用来诱导血小板的活化和聚集,促进肺癌的生长,因此鬼臼素可作为一种血小板活化和聚集的关键调节因子[31]。肺癌细胞高表达组织因子,其作为启动外源性凝血级联反应的重要因子,通过与因子VIIa结合来激活下游信号传导,促进凝血酶生成,从而导致血小板活化、纤维蛋白沉积。最近XIA等[32]报道组织因子在非小细胞肺癌肿瘤生长、转移和侵袭中的作用,表明血浆组织因子水平可作为预测非小细胞肺癌患者预后的有效非侵入性标志物。此外,癌症诱导NETs的形成间接激活血小板,从而导致血小板聚集和血栓形成。 2.3.2 活化血小板在肺癌中的作用 血小板活化后聚集在肿瘤细胞表面形成肿瘤细胞-血小板聚集体,并在肿瘤表面形成一层保护网,使循环肿瘤细胞免受血液剪切应力的攻击,从而在血管内停滞和存活[33]。ZHOU等[34]还提出血小板保护肿瘤细胞逃脱免疫系统的攻击。NK细胞是细胞毒性免疫细胞,可杀死主要组织相容性复合物Ⅰ类分子表达低或无表达的靶细胞。在NK细胞存在的情况下,血小板活化是肿瘤细胞存活所必需的。在肿瘤微环境中,血小板活化后将自身的主要组织相容性复合物Ⅰ类分子转移到循环肿瘤细胞的表面,因此当含有这些分子的循环肿瘤细胞被NK细胞识别时,后者将忽略它们,导致免疫逃逸。除此之外,也有学者表明活化血小板释放的转化生长因子β下调了NK细胞上的人自然杀伤细胞家族2成员D的表达,以抑制NK细胞的抗肿瘤活性[35]。转化生长因子β还降低了NK细胞上CD226及其配体CD155在循环肿瘤细胞表面的表达,从而减少了它们之间的接触。血小板衍生生长因子则直接抑制NK细胞的细胞毒性[36]。肿瘤细胞在活化血小板的保护下得以存活,从而为后续的一系列进展提供先决条件。活化血小板能介导肿瘤细胞与血管内皮细胞的黏附,促进肿瘤细胞在血管内的迁移和停滞。血小板表达多种黏附分子,如整合素(包括αIIbβ3、αVβ3、α2β1、α6β1等)、选择素(P选择素)、C型凝集素受体家族蛋白、富含亮氨酸的糖蛋白等,这些黏附分子在血小板活化后大量表达或活性增强,支持循环肿瘤细胞在脉管系统内的阻滞。在循环肿瘤细胞稳定黏附在转移部位的内皮细胞后,血小板继续促进肿瘤细胞的跨内皮迁移、侵袭以及随后的存活和增殖。为了进行转移,肿瘤细胞必须外渗。血小板活化后释放的多种活性递质促进肿瘤细胞外渗,如ATP、组胺、基质金属蛋白酶、溶血磷脂酸等。此外,上皮-间充质转化在外渗中发挥重要作用。血小板活化后分泌转化生长因子β1,转化生长因子β1激活肿瘤细胞中的转化生长因子β/Smad通路,同时血小板-肿瘤细胞直接接触时NF-κB通路也激活,两条通路协同作用,诱导肿瘤细胞表型从上皮细胞向间充质表型转变,赋予肿瘤细胞干细胞样特性,从而增加肿瘤侵袭性和转移性[37]。众所周知,随着肿瘤细胞的生长,它们必须募集新的血管。新生的血管即可以为肿瘤提供氧气营养,又为肿瘤细胞的转移提供通道。血小板α颗粒是促血管生成因子的主要储存库,包括血管内皮生长因子、血小板衍生生长因子和碱性成纤维细胞生长因子[38],这些因子在血小板活化过程中释放。LI等[39]报道与血小板相互作用的肺癌细胞可能通过血管内皮生长因子/血管内皮生长因子受体2信号通路在肺癌血管生成中发挥关键作用。同时,活化血小板在保持肿瘤血管稳态中也具有重要贡献。在一项荷瘤小鼠研究中,HO-TIN-NOé等[40]发现血小板耗竭导致肿瘤血管不稳定,在诱导血小板减少后30 min开始出现瘤内出血。同时,他们比较了输注静息血小板和脱颗粒血小板预防瘤内出血的能力,发现静息血小板可防止血小板减少症诱导的肿瘤出血,而循环脱颗粒血小板则不能,这表明预防瘤内出血依赖于血小板颗粒内容物的分泌。除了上述作用外,LABELLE等[41]提出了血小板引导早期转移性生态位形成,研究表明粒细胞快速募集到肿瘤细胞以形成“早期转移性生态位”需要血小板与肿瘤细胞接触时分泌CXCL5和CXCL7趋化因子来介导。如果阻断CXCL5/7受体CXCR2或者血小板和粒细胞的短暂耗竭可防止早期转移性生态位的形成,并显著抑制转移和进展。 血小板和肿瘤细胞表面存在多种多样受体分子,二者相互作用,见图6[42]。 "
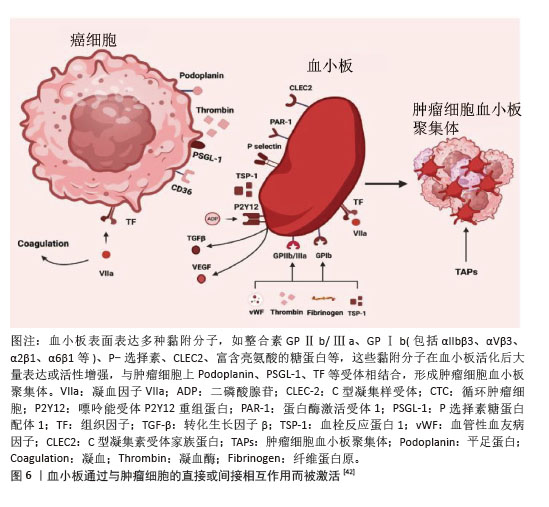
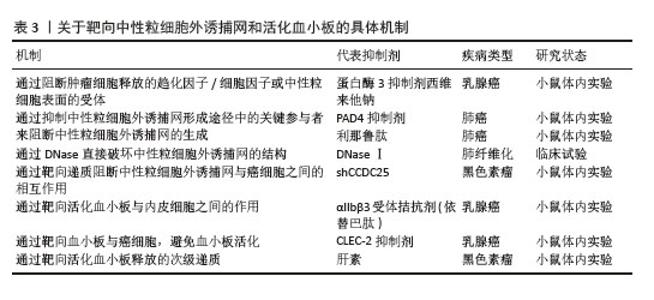
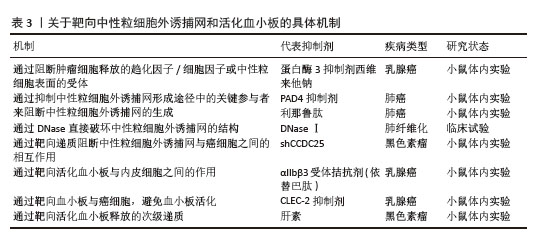
2.3.3 活化血小板相关物质在肺癌中的表达 P-选择素(CD62P)是选择素家族中的一种亚型,主要存在于血小板α颗粒和血管内皮细胞的Weibel-Palade体中。通常P-选择素在静息状态下缺失或持续低表达,当血小板受到刺激活化时,P-选择素(CD62P)通过膜融合迅速转移到血小板表面。因此,P-选择素常作为血小板活化的标志物。P-选择素在血小板与肿瘤细胞的结合以及肿瘤细胞与血管内皮细胞的黏附中起重要作用,这些特定过程是肿瘤转移的必经步骤。GONG等[43]发现P-选择素在肺腺癌中的表达高于肺鳞状细胞癌,癌旁组织高于远处组织;同时,非小细胞肺癌患者外周血中也发现血小板计数和P-选择素显著升高;与Ⅰ+Ⅱ期和Ⅲ期患者相比,Ⅳ期患者血小板升高显著。活化的血小板-肺腺癌细胞复合物通过P-选择素促进血管内皮表面肺腺癌细胞的捕获,由此促进肿瘤细胞的血行转移,这表明血小板活化、P-选择素表达与非小细胞肺癌特别是肺腺癌的血行转移之间存在相关性。整合素αIIbβ3在肺癌细胞中高表达。整合素αIIbβ3是血小板膜表面重要的糖蛋白受体家族分子之一,其功能主要是介导多种细胞之间以及细胞与细胞外基质之间的黏附,在血小板表面以非活性结构状态大量表达。当血小板活化时,整合素αIIbβ3由低亲和力状态转变为高亲和力状态,导致血小板之间的聚集[44]。任鹏飞等[45]证明了血小板衍生生长因子BB在非小细胞肺癌中高表达,并且与骨转移有关。血管内皮生长因子和碱性成纤维细胞生长因子是血管生成的关键调节因子。HU等[46]在一项分析中报道了对于可手术的肺癌患者,碱性成纤维细胞生长因子过表达是预后较差的潜在指标。 2.4 NETs和活化血小板 2.4.1 NETs和活化血小板相互作用 活化血小板作为NETs的激活物之一,直接诱导NETs的形成。血小板活化后将P-选择素转移到表面,与中性粒细胞表面受体P-选择素糖蛋白配体1结合,促进中性粒细胞-血小板黏附、中性粒细胞活化及随后的NETs释放[47]。活化血小板向中性粒细胞呈递高迁移率组蛋白1,诱导中性粒细胞自噬并促进NETs的形成。在高迁移率组蛋白1竞争性拮抗剂存在下,NETs的形成受阻[48]。NETs为血小板活化和聚集及血栓形成提供支架。当小鼠注射NETs抑制剂时,NETs降解后没有出现血小板聚集体,这表明NETs是血小板活化和血栓形成所必需的底物[49]。JUNG等[50]提出NETs中所含的DNA成分是NETs相关凝血酶生成和随后癌症相关血栓形成现象的触发因素。血小板的黏附和活化并不完全是由于NETs的DNA成分,因为用DNAseⅠ对NETs进行预处理并没有完全消除血小板的黏附和扩散,这表明NETs的蛋白质成分也有助于血小板黏附和活化[51],如组蛋白、中性粒细胞弹性蛋白酶、组织蛋白酶G和髓过氧化物酶。组蛋白通过激活血小板上Toll样受体2和Toll样受体4来诱导血小板的活化,并驱动血浆凝血酶生成。中性粒细胞弹性蛋白酶和组织蛋白酶G通过抑制组织因子通路抑制剂增强组织因子和凝血因子Ⅻ表达,从而促进了凝血过程和随后的血小板活化[52]。髓过氧化物酶与人血小板质膜结合,诱导肌动蛋白细胞骨架重组,胞质内Ca2+浓度升高,从而触发Ca2+依赖性信号传导过程,导致激动剂诱导的血小板聚集和活化[53]。综上得出,NETs和血小板之间的相互作用是双向的,活化的血小板是NETs的有效诱导剂,而NETs反过来可进一步增强血小板的活化,两者之间相互作用共同促进肿瘤的进展。REN等[47]测试了NETs与有或没有血小板活化的肿瘤细胞之间的作用,将MC38癌细胞、NETs与肝缺血再灌注损伤小鼠模型及假手术小鼠模型中分离的血小板共培养,发现与对照假手术小鼠(无血小板活化)相比,缺血再灌注损伤小鼠(血小板被激活)捕获的肿瘤细胞更多。在肝脏缺血再灌注损伤小鼠模型中,血小板被激活,促进了血小板-肿瘤细胞聚集体的形成,促进了NETs介导的对血小板-肿瘤细胞聚集体的捕获,然后刺激肿瘤细胞的迁移导致最后的远处转移,这表明在血小板活化的帮助下,NETs介导的肿瘤的侵袭力和转移力增强。考虑到NETs和活化血小板在肺癌中起到相辅相成、共同促进的作用,联合标志物诊断可能比单一标志物诊断更有效。在最近一项100例急性心肌梗死患者病例对照研究中,HALLY等[54]使用多变量回归模型评估复合生物标志物(NETs和血小板活化的生物标志物)评分,发现这些复合生物标志物评分是1年主要不良心脑血管事件的独立预测因子,与主要不良心脑血管事件的相关性最强,表明联合具有紧密关联但不同生物途径的生物标志物比单独使用任何一种生物标志物更有效。但是目前在肺癌方面未发现将二者联合起来用于肺癌的诊断、预后监测的研究,期望未来能在此领域探索。 2.4.2 NETs和活化血小板在肺癌临床治疗中的潜力 肺癌的治疗手段多种多样,包括手术切除、化疗、放疗、免疫治疗及靶向治疗等,但肺癌的预后仍较差,生存率较低,这就迫切需要研制治疗肺癌的更高效手段。NETs和活化血小板促进癌细胞存活、转移及生长。因此开发精确的抗血小板活化治疗方案及选择性靶向NETs显得尤为重要,见表3。"
| [1] BRAY F, LAVERSANNE M, SUNG H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. [2] ZHENG RS, CHEN R, HAN BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 2024;46(3):221-231. [3] FANG R, ZHU Y, KHADKA VS, et al. The Evaluation of Serum Biomarkers for Non-small Cell Lung Cancer (NSCLC) Diagnosis. Front Physiol. 2018;9:1710. [4] MILLER KD, SIEGEL RL, LIN CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289. [5] BRINKMANN V, REICHARD U, GOOSMANN C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-1535. [6] FUCHS TA, ABED U, GOOSMANN C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231-241. [7] BERGER-ACHITUV S, BRINKMANN V, ABED UA, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. [8] LI X, HU L, NAEEM A, et al. Neutrophil Extracellular Traps in Tumors and Potential Use of Traditional Herbal Medicine Formulations for Its Regulation. Int J Nanomedicine. 2024; 19:2851-2877. [9] WIGERBLAD G, KAPLAN MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. 2023;23(5):274-288. [10] XIAO Y, CONG M, LI J, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423-437.e7. [11] LI Y, YANG Y, GAN T, et al. Extracellular RNAs from lung cancer cells activate epithelial cells and induce neutrophil extracellular traps. Int J Oncol. 2019;55(1):69-80. [12] ZHOU X, WU C, WANG X, et al. Tumor cell-released autophagosomes (TRAPs) induce PD-L1-decorated NETs that suppress T-cell function to promote breast cancer pulmonary metastasis. J Immunother Cancer. 2024;12(6):e009082. [13] TEIJEIRA Á, GARASA S, GATO M, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52(5):856-871.e8. [14] KALTENMEIER C, YAZDANI HO, MORDER K, et al. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front Immunol. 2021;12: 785222. [15] PENG YP, ZHANG JJ, LIANG WB, et al. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC Cancer. 2014;14:738. [16] COOLS-LARTIGUE J, SPICER J, MCDONALD B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013; 123(8):3446-3458. [17] NAJMEH S, COOLS-LARTIGUE J, RAYES RF, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer. 2017;140(10):2321-2330. [18] YANG L, LIU Q, ZHANG X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020; 583(7814):133-138. [19] WANG Y, LIU F, CHEN L, et al. Neutrophil Extracellular Traps (NETs) Promote Non-Small Cell Lung Cancer Metastasis by Suppressing lncRNA MIR503HG to Activate the NF-κB/NLRP3 Inflammasome Pathway. Front Immunol. 2022;13:867516. [20] ALBRENGUES J, SHIELDS MA, NG D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227. [21] DEMERS M, WONG SL, MARTINOD K, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016;5(5):e1134073. [22] YAZDANI HO, ROY E, COMERCI AJ, et al. Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res. 2019;79(21): 5626-5639. [23] HOUGHTON AM, RZYMKIEWICZ DM, JI H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219-223. [24] DEMERS M, KRAUSE DS, SCHATZBERG D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109(32):13076-13081. [25] OKLU R, SHETH RA, WONG KHK, et al. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S140-S149. [26] RAYES RF, MOUHANNA JG, NICOLAU I, et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5(16):e128008. [27] ZUO Y, LENG G, LENG P. Identification and validation of molecular subtype and prognostic signature for lung adenocarcinoma based on neutrophil extracellular traps. Pathol Oncol Res. 2023;29:1610899. [28] FANG C, LIU F, WANG Y, et al. A innovative prognostic symbol based on neutrophil extracellular traps (NETs)-related lncRNA signature in non-small-cell lung cancer. Aging (Albany NY). 2021;13(13):17864-17879. [29] WANG M, LV X, WANG Y, et al. Biomarkers of peripheral blood neutrophil extracellular traps in the diagnosis and progression of malignant tumors. Cancer Med. 2024;13(3):e6935. [30] ANSARI D, ANSARI D, ANDERSSON R, et al. Pancreatic cancer and thromboembolic disease, 150 years after Trousseau. Hepatobiliary Surg Nutr. 2015;4(5):325-335. [31] MIYATA K, TAKEMOTO A, OKUMURA S, et al. Podoplanin enhances lung cancer cell growth in vivo by inducing platelet aggregation. Sci Rep. 2017;7(1):4059. [32] XIA Q, ZHANG X, CHEN Q, et al. Down-regulation of tissue factor inhibits invasion and metastasis of non-small cell lung cancer. J Cancer. 2020;11(5):1195-1202. [33] LABELLE M, HYNES RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091-1099. [34] ZHOU L, ZHANG Z, TIAN Y, et al. The critical role of platelet in cancer progression and metastasis. Eur J Med Res. 2023;28(1):385. [35] WIRTZ D, KONSTANTOPOULOS K, SEARSON PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11(7):512-522. [36] LIU Y, ZHANG Y, DING Y, et al. Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules. Crit Rev Oncol Hematol. 2021;167:103502. [37] TONG H, LI K, ZHOU M, et al. Coculture of cancer cells with platelets increases their survival and metastasis by activating the TGFβ/Smad/PAI-1 and PI3K/AKT pathways. Int J Biol Sci. 2023;19(13):4259-4277. [38] BRAUN A, ANDERS HJ, GUDERMANN T, et al. Platelet-Cancer Interplay: Molecular Mechanisms and New Therapeutic Avenues. Front Oncol. 2021;11:665534. [39] LI B, ZHU T, WU X, et al. The crosstalk between lung cancer cells and platelets promotes tumor angiogenesis in vivo and in vitro. J Cancer Res Clin Oncol. 2023;149(7):3495-3511. [40] HO-TIN-NOÉ B, GOERGE T, CIFUNI SM, et al. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68(16):6851-6858. [41] LABELLE M, BEGUM S, HYNES RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111(30):E3053-3061. [42] RASKOV H, ORHAN A, AGERBÆK MØ, et al. The impact of platelets on the metastatic potential of tumour cells. Heliyon. 2024; 10(14):e34361. [43] GONG L, MI HJ, ZHU H, et al. P-selectin-mediated platelet activation promotes adhesion of non-small cell lung carcinoma cells on vascular endothelial cells under flow. Mol Med Rep. 2012;5(4):935-942. [44] HUANG J, LI X, SHI X, et al. Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting. J Hematol Oncol. 2019;12(1):26. [45] 任鹏飞,王弦,李晶晶,等.非小细胞肺癌中PDGF-BB表达及其与骨转移的相关性[J].临床与实验病理杂志,2021,37(6):669-673. [46] HU M, HU Y, HE J, et al. Prognostic Value of Basic Fibroblast Growth Factor (bFGF) in Lung Cancer: A Systematic Review with Meta-Analysis. PLoS One. 2016;11(1):e0147374. [47] REN J, HE J, ZHANG H, et al. Platelet TLR4-ERK5 Axis Facilitates NET-Mediated Capturing of Circulating Tumor Cells and Distant Metastasis after Surgical Stress. Cancer Res. 2021;81(9): 2373-2385. [48] ZHOU J, YANG Y, GAN T, et al. Lung cancer cells release high mobility group box 1 and promote the formation of neutrophil extracellular traps. Oncol Lett. 2019;18(1):181-188. [49] FUCHS TA, BRILL A, DUERSCHMIED D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010; 107(36):15880-15885. [50] JUNG HS, GU J, KIM JE, et al. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS One. 2019;14(4): e0216055. [51] ABDOL RAZAK N, ELASKALANI O, METHAROM P. Pancreatic Cancer-Induced Neutrophil Extracellular Traps: A Potential Contributor to Cancer-Associated Thrombosis. Int J Mol Sci. 2017;18(3):487. [52] MASSBERG S, GRAHL L, VON BRUEHL ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887-896. [53] GORUDKO IV, SOKOLOV AV, SHAMOVA EV, et al. Myeloperoxidase modulates human platelet aggregation via actin cytoskeleton reorganization and store-operated calcium entry. Biol Open. 2013;2(9):916-923. [54] HALLY KE, PARKER OM, BRUNTON-O’SULLIVAN MM, et al. Linking Neutrophil Extracellular Traps and Platelet Activation: A Composite Biomarker Score for Predicting Outcomes after Acute Myocardial Infarction. Thromb Haemost. 2021;121(12):1637-1649. [55] TARRANT BJ, SNELL G, IVULICH S, et al. Dornase alfa during lower respiratory tract infection post-lung transplantation: a randomized controlled trial. Transpl Int. 2019;32(6):603-613. [56] ZENG X, LI J, PEI L, et al. Didang decoction attenuates cancer-associated thrombosis by inhibiting PAD4-dependent NET formation in lung cancer. Pulm Circ. 2024;14(4):e12454. [57] LI M, LIN C, DENG H, et al. A Novel Peptidylarginine Deiminase 4 (PAD4) Inhibitor BMS-P5 Blocks Formation of Neutrophil Extracellular Traps and Delays Progression of Multiple Myeloma. Mol Cancer Ther. 2020; 19(7):1530-1538. [58] ETULAIN J, MARTINOD K, WONG SL, et al. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126(2):242-246. [59] CHEN D, LIANG H, HUANG L, et al. Liraglutide enhances the effect of checkpoint blockade in lung and liver cancers through the inhibition of neutrophil extracellular traps. FEBS Open Bio. 2024;14(8):1365-1377. [60] ROTHWELL PM, PRICE JF, FOWKES FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602-1612. [61] SUZUKI-INOUE K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134(22):1912-1918. [62] ZHAO F, LI L, GUAN L, et al. Roles for GP IIb/IIIa and αvβ3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett. 2014; 344(1):62-73. [63] QI CL, WEI B, YE J, et al. P-selectin-mediated platelet adhesion promotes the metastasis of murine melanoma cells. PLoS One. 2014; 9(3):e91320. |
| [1] | Lai Jiaming, , Song Yuling, Chen Zixi, Wei Jinghuan, Cai Hao, , Li Guoquan, . Screening of diagnostic markers for endothelial cell Senescence in mice with radiation-induced heart disease and analysis of immune infiltration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1450-1463. |
| [2] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [3] | Guan Yujie, Zhao Bin. Application and prospect of artificial intelligence in screening and diagnosis of scoliosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 721-730. |
| [4] | Zhang Qian, Wang Fuxia, Wang Wen, Zhang Kun. Characteristic analysis of nanogel composite system and its application strategies in visualization of diagnostic imaging and therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 480-488. |
| [5] | Deng Keqi, Li Guangdi, Goswami Ashutosh, Liu Xingyu, He Xiaoyong. Screening and validation of Hub genes for iron overload in osteoarthritis based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1972-1980. |
| [6] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [7] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [8] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [9] | Yang Dingyan, Yu Zhenqiu, Yang Zhongyu. Machine learning-based analysis of neutrophil-associated potential biomarkers for acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7909-7920. |
| [10] | Zhang Yixuan, Li Dongna, Liu Chunyan. Pathological processes, inflammatory responses, and related biomarkers of periodontitis: a multi-omics analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7601-7610. |
| [11] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [12] |
Zhou Rulin, Hu Yuanzheng, Wang Zongqing, Zhou Guoping, Zhang Baochao, Xu Qian, Bai Fanghui.
Exploration of biomarkers for moyamoya disease and analysis of traditional Chinese medicine targets#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6927-6938.
|
| [13] | Song Haoran, Zhang Yuqiang, Gu Na, Zhi Xiaodong, Wang Wei. Visualization analysis of artificial intelligence in bone trauma research based on Citespace [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 493-502. |
| [14] | Hao Maochen, Ma Chao, Liu Kai, Liu Kexin, Meng Lingting, Wang Xingru, Wang Jianzhong. Bioinformatics screening of key genes for endoplasmic reticulum stress in osteoarthritis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5632-5641. |
| [15] | Wang Lei, Wang Baiyan, Zhou Chunguang, Ren Xiaoyun, Dai Yueyou, Feng Shuying. Role of different cell-derived exosomal miRNAs in progression, diagnosis, and prognosis of gastric cancer [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5434-5442. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||