Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 120-129.doi: 10.12307/2026.538
Previous Articles Next Articles
Alpha-ketoglutarate engineered small extracellular vesicles delay skin aging
Wu Zhijing1, 2, Li Jiali1, 2, Zhang Jiaxin1, 2, Wang Tangrong1, 2, Zheng Yuzhou1, 2, Sun Zixuan1, 2
- 1Department of Laboratory Medicine, Medical College of Jiangsu University, Zhenjiang 212013, Jiangsu Province, China; 2Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application, Zhenjiang 212013, Jiangsu Province, China
-
Received:2024-11-15Accepted:2025-01-21Online:2026-01-08Published:2025-07-02 -
Contact:Sun Zixuan, MD, Associate professor, Master’s supervisor, Department of Laboratory Medicine, Medical College of Jiangsu University, Zhenjiang 212013, Jiangsu Province, China; Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application, Zhenjiang 212013, Jiangsu Province, China -
About author:Wu Zhijing, Master candidate, Department of Laboratory Medicine, Medical College of Jiangsu University, Zhenjiang 212013, Jiangsu Province, China; Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application, Zhenjiang 212013, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 82003379 (to SZX)
CLC Number:
Cite this article
Wu Zhijing, Li Jiali, Zhang Jiaxin, Wang Tangrong, Zheng Yuzhou, Sun Zixuan. Alpha-ketoglutarate engineered small extracellular vesicles delay skin aging[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 120-129.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
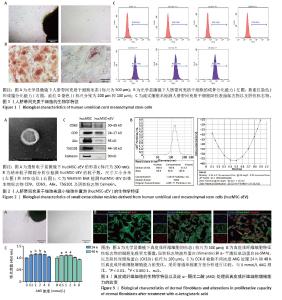
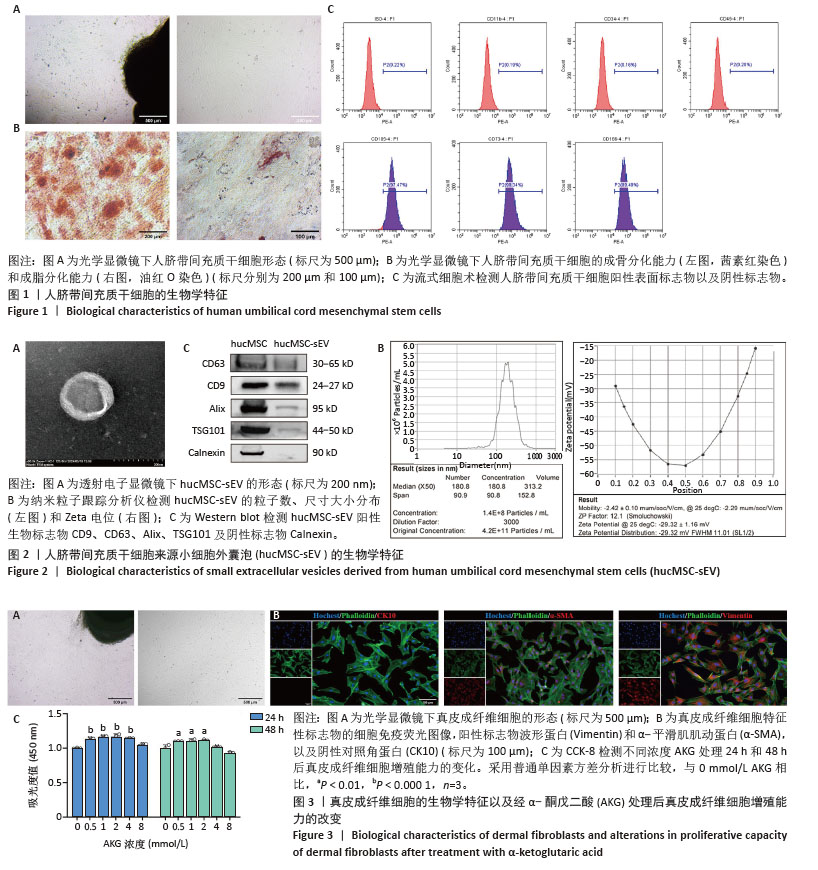
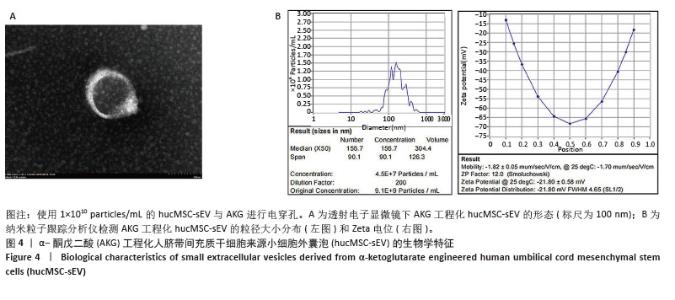
2.1 人脐带间充质干细胞及hucMSC-sEV的生物学特征 分离培养的原代人脐带间充质干细胞从组织块周围爬出,细胞形态呈梭状,细胞状态良好呈漩涡状生长,见图1A;经过成骨和成脂培养基诱导分化后进行茜素红或油红O染色,可见明显的钙结节和脂滴,表明人脐带间充质干细胞成骨、成脂分化能力良好,见图1B;流式细胞术结果表明,人脐带间充质干细胞高表达阳性标志物CD105、CD73、CD166,阴性标志物CD11b、 CD34、CD45和ISO几乎不表达,见图1C,符合间充质干细胞的生物学特征。 收集人脐带间充质干细胞上清液,进行差速-超速离心法得到hucMSC-sEV,在透射电镜下观察到hucMSC-sEV有典型的囊泡膜结构并且呈茶托状,见图2A;纳米粒子跟踪分析仪检测粒径分布在180.8 nm处,Zeta电位为(-29.32±1.16) mV,见图2B;Western blot结果显示,hucMSC-sEV表达小细胞外囊泡特征性标志物CD63、CD9、Alix、TSG101,不表达阴性标志物Calnexin,见图2C。 2.2 AKG对真皮成纤维细胞增殖能力的影响 分离培养的真皮成纤维细胞从大鼠乳鼠的皮肤组织块中爬出,细胞形态呈短梭状,细胞状态良好呈漩涡状生长,见图3A;细胞免疫荧光结果显示,真皮成纤维细胞表达特征性标志物波形蛋白和α-平滑肌肌动蛋白,不表达阴性标志物即表皮标志物角蛋白10,见图3B,符合真皮成纤维细胞的生物学特征。分别将0.5,1,2,4,8 mmol/L的AKG与真皮成纤维细胞共孵育24 h和48 h,CCK-8结果显示AKG没有抑制真皮成纤维细胞的增殖,表明AKG对真皮成纤维细胞无毒性,见图3C,由于在传统的电穿孔过程中投入的药物量会有一定的损失,同时通过参考相关文献[35],最终选择2 mmol/L浓度进行后续实验。 "
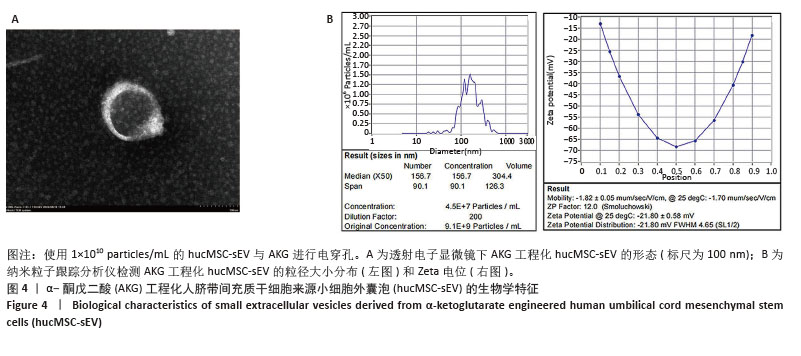
2.3 AKG工程化hucMSC-sEV的构建与鉴定 使用电穿孔技术,分别将2 mmol/L的AKG载入5×109 particles/mL,1×1010 particles/mL和5×1010 particles/mL的hucMSC-sEV中,在透射电镜下观察到AKG工程化hucMSC-sEV囊泡膜结构完整并呈茶托状,见图4A;纳米粒子跟踪分析仪检测AKG工程化hucMSC-sEV的粒子分布和Zeta电位,与hucMSC-sEV相比均无明显变化,表明电穿孔对小细胞外囊泡几乎无损伤作用,见图4B。使用高效液相色谱技术检测AKG工程化hucMSC-sEV的包封率,得到标准曲线为Y=1 613 593.088X+ 21 958.278,R2=0.999 9,表明AKG的浓度在0-5 mmol/L范围内线性关系良好。通过公式计算5×109,1×1010,5× 1010 particles/mL的hucMSC-sEV与AKG进行电穿孔后相应的包封率分别为(44.93±0.97)%,(45.38±4.36)%和(40.13±2.31)%,结果显示当小细胞外囊泡粒子数在1×1010 particles/mL时包封率最高,因此选择此浓度进行后续实验。 "
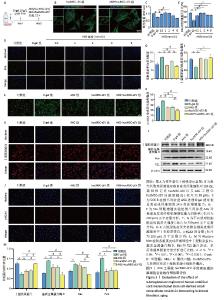
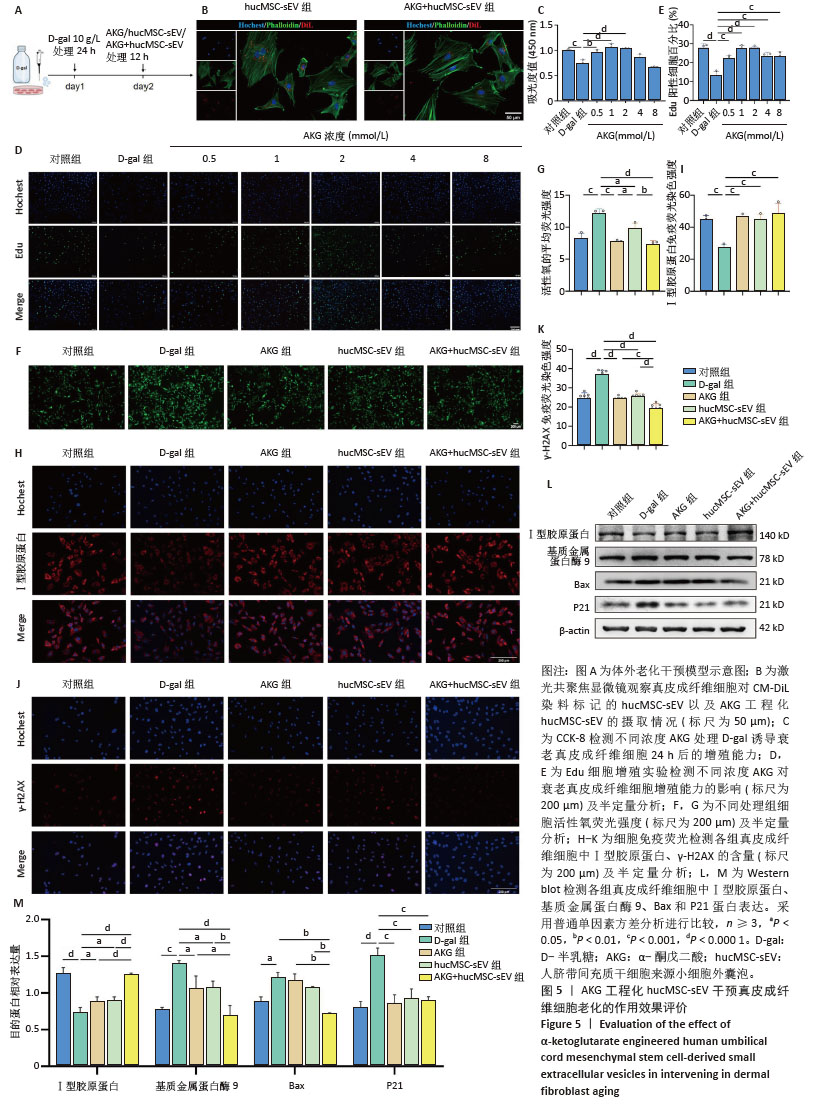
2.4 AKG工程化hucMSC-sEV延缓D-半乳糖诱导的真皮成纤维细胞衰老进程 建立真皮成纤维细胞老化模型,见图5A,在D-半乳糖处理24 h后,向细胞中分别加入AKG或hucMSC-sEV或AKG工程化hucMSC-sEV处理12 h进行后续实验。在共聚焦显微镜下观察到hucMSC-sEV与真皮成纤维细胞共孵育12 h后能被有效摄取,而AKG工程化hucMSC-sEV组的摄取效率要略低于hucMSC-sEV组,可能与电穿孔造成小细胞外囊泡数量上的损失有关,见图5B。 2.4.1 AKG工程化hucMSC-sEV促进衰老真皮成纤维细胞的增殖 通过CCK-8法来评估不同浓度AKG对D-半乳糖诱导的衰老真皮成纤维细胞增殖的影响。使用0.5,1,2,4,8 mmol/L AKG与真皮成纤维细胞共孵育,CCK-8结果显示AKG浓度在2 mmol/L以内时,对衰老的真皮成纤维细胞有促增殖作用,且当浓度为1 mmol/L和2 mmol/L时效果最为显著,Edu细胞增殖实验也具有相同的结果,见图5C-E,结合AKG对真皮成纤维细胞的毒性实验,以及药物在电穿孔过程中存在损失,最终选取2 mmol/L进行后续实验。 2.4.2 AKG工程化hucMSC-sEV降低衰老真皮成纤维细胞氧化应激水平 使用活性氧评估不同处理组衰老真皮成纤维细胞内氧化应激水平,结果显示,与D-半乳糖组相比,各处理组显著抑制了细胞内活性氧的产生,降低了氧化应激水平,其中AKG工程化hucMSC-sEV组效果更优,见图5F-G。 2.4.3 AKG工程化hucMSC-sEV减少真皮成纤维细胞的胶原蛋白流失并降低DNA损伤水平 Western blot结果显示,与D-半乳糖组相比,AKG工程化hucMSC-sEV组能有效增加Ⅰ型胶原蛋白表达,并下调基质金属蛋白酶9表达,在一定程度上抑制了胶原蛋白的降解,同时下调Bax蛋白表达,有效抑制了细胞凋亡,此外衰老相关标志物P21表达水平也显著下调。细胞免疫荧光结果也证实,AKG组、hucMSC-sEV组和AKG工程化hucMSC-sEV组均能提升Ⅰ型胶原蛋白表达水平,下调DNA双链断裂标志物γ-H2AX表达水平,表明经处理后,衰老真皮成纤维细胞的胶原蛋白含量有所恢复,DNA断裂引起的损伤减轻,其中AKG工程化hucMSC-sEV组的效果最好,见图5H-M。"
| [1] FRANCO AC, AVELEIRA C, CAVADAS C. Skin senescence: mechanisms and impact on whole-body aging. Trends Mol Med. 2022;28(2):97-109. [2] CSEKES E, RAČKOVÁ L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int J Mol Sci. 2021;22(23):12641. [3] GRIFFITHS TW, WATSON REB, LANGTON AK. Skin ageing and topical rejuvenation strategies. Br J Dermatol. 2023;189(Suppl 1):i17-i23. [4] BLAIR MJ, JONES JD, WOESSNER AE, et al. Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv Wound Care (New Rochelle). 2020;9(3):127-143. [5] HÖHN A, WEBER D, JUNG T, et al. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017;11:482-501. [6] HARLEY CB, FUTCHER AB, GREIDER CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458-460. [7] PEZONE A, OLIVIERI F, NAPOLI MV, et al. Inflammation and DNA damage: cause, effect or both. Nat Rev Rheumatol. 2023;19(4):200-211. [8] BHARATH LP, AGRAWAL M, MCCAMBRIDGE G, et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020;32(1): 44-55.e6. [9] ANSARY TM, HOSSAIN MR, KAMIYA K, et al. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int J Mol Sci. 2021;22(8):3974. [10] LOVELL CR, SMOLENSKI KA, DUANCE VC, et al. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br J Dermatol. 1987;117(4):419-428. [11] WANG H, WEI S, XUE X, et al. Adipose stem cells’ antagonism in glycosylation of D-galactose-induced skin aging of nude mice and its skin recovery function. Int J Immunopathol Pharmacol. 2016;29(3): 376-385. [12] CRISAN M, TAULESCU M, CRISAN D, et al. Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PLoS One. 2013;8(10):e75003. [13] WANG L, JIANG Y, ZHAO C. The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp Dermatol. 2024;33(4):e15065. [14] MAURELLI M, GISONDI P, GIROLOMONI G. Advanced Glycation End Products and Psoriasis. Vaccines (Basel). 2023;11(3):617. [15] THÉRY C, WITWER KW, AIKAWA E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [16] ZHANG H, XIAO X, WANG L, et al. Human adipose and umbilical cord mesenchymal stem cell-derived extracellular vesicles mitigate photoaging via TIMP1/Notch1. Signal Transduct Target Ther. 2024; 9(1):294. [17] SUN Z, WANG T, HOU X, et al. Mesenchymal stromal cells-derived small extracellular vesicles protect against UV-induced photoaging via regulating pregnancy zone protein. Stem Cells Transl Med. 2024; 13(11):1129-1143. [18] RÄDLER J, GUPTA D, ZICKLER A, et al. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol Ther. 2023;31(5):1231-1250. [19] 张咪,吴赛璇,董明,等.新型纳米递送系统:工程化小细胞外囊泡[J].中国组织工程研究,2022,26(27):4417-4422. [20] WU P, ZHANG B, OCANSEY DKW, et al. Extracellular vesicles: A bright star of nanomedicine. Biomaterials. 2021;269:120467. [21] HERRMANN IK, WOOD MJA, FUHRMANN G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021; 16(7):748-759. [22] YUAN Y, ZHU C, WANG Y, et al. α-Ketoglutaric acid ameliorates hyperglycemia in diabetes by inhibiting hepatic gluconeogenesis via serpina1e signaling. Sci Adv. 2022;8(18):eabn2879. [23] 吴楠.α-酮戊二酸在果蝇抗衰老中的作用及其机制研究[D].雅安:四川农业大学,2017. [24] CHIN RM, FU X, PAI MY, et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014; 510(7505):397-401. [25] YANG F, ZHOU Z, GUO M, et al. The study of skin hydration, anti-wrinkles function improvement of anti-aging cream with alpha-ketoglutarate. J Cosmet Dermatol. 2022;21(4):1736-1743. [26] SON ED, CHOI GH, KIM H, et al. Alpha-ketoglutarate stimulates procollagen production in cultured human dermal fibroblasts, and decreases UVB-induced wrinkle formation following topical application on the dorsal skin of hairless mice. Biol Pharm Bull. 2007;30(8): 1395-1399. [27] ASADI SHAHMIRZADI A, EDGAR D, LIAO CY, et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020;32(3):447-456.e6. [28] LIU S, HE L, YAO K. The Antioxidative Function of Alpha-Ketoglutarate and Its Applications. Biomed Res Int. 2018;2018:3408467. [29] SALMINEN A, KAARNIRANTA K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11(2):230-241. [30] BAYLIAK MM, LUSHCHAK VI. Pleiotropic effects of alpha-ketoglutarate as a potential anti-ageing agent. Ageing Res Rev. 2021;66:101237. [31] KOMURO H, AMINOVA S, LAURO K, et al. Advances of engineered extracellular vesicles-based therapeutics strategy. Sci Technol Adv Mater. 2022;23(1):655-681. [32] MUKHOPADHYA A, TSIAPALIS D, MCNAMEE N, et al. Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics. 2023;15(3):718. [33] CHEN C, LI Y, WANG Q, et al. Single-particle assessment of six different drug-loading strategies for incorporating doxorubicin into small extracellular vesicles. Anal Bioanal Chem. 2023;415(7):1287-1298. [34] TIAN J, HAN Z, SONG D, et al. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int J Nanomedicine. 2023;18:7923-7940. [35] WANG Y, DENG P, LIU Y, et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat Commun. 2020;11(1):5596. [36] LEE CM, WATSON REB, KLEYN CE. The impact of perceived stress on skin ageing. J Eur Acad Dermatol Venereol. 2020;34(1):54-58. [37] SHIN SH, LEE YH, RHO NK, et al. Skin aging from mechanisms to interventions: focusing on dermal aging. Front Physiol. 2023;14: 1195272. [38] KRUTMANN J, SCHIKOWSKI T, MORITA A, et al. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J Invest Dermatol. 2021;141(4S):1096-1103. [39] KOHL E, STEINBAUER J, LANDTHALER M, et al. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25(8):873-884. [40] MARTINI H, PASSOS JF. Cellular senescence: all roads lead to mitochondria. FEBS J. 2023;290(5):1186-1202. [41] SREEDHAR A, AGUILERA-AGUIRRE L, SINGH KK. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020;11(6):444. [42] POLJŠAK B, DAHMANE RG, GODIĆ A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat. 2012; 21(2):33-36. [43] LEE H, HONG Y, KIM M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int J Mol Sci. 2021;22(22):12489. [44] UMBAYEV B, ASKAROVA S, ALMABAYEVA A, et al. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid Med Cell Longev. 2020; 2020:7145656. [45] KUMAR H, BHARDWAJ K, VALKO M, et al. Antioxidative potential of Lactobacillus sp. in ameliorating D-galactose-induced aging. Appl Microbiol Biotechnol. 2022;106(13-16):4831-4843. [46] WEI H, LI L, SONG Q, et al. Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav Brain Res. 2005;157(2):245-251. [47] AZMAN KF, ZAKARIA R. D-Galactose-induced accelerated aging model: an overview. Biogerontology. 2019;20(6):763-782. [48] DU XL, EDELSTEIN D, ROSSETTI L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97(22): 12222-12226. [49] GYANWALI B, LIM ZX, SOH J, et al. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol Metab. 2022;33(2):136-146. [50] WIKLANDER OPB, BRENNAN MÁ, LÖTVALL J, et al. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):eaav8521. |
| [1] | Haonan Yang, Zhengwei Yuan, Junpeng Xu, Zhiqi Mao, Jianning Zhang. Preliminary study on the mechanisms and efficacy of deep brain stimulation in treating depression [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-9. |
| [2] | Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling. Applications and limitations of antioxidant nanomaterials in oral cavity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2044-2053. |
| [3] |
Dong Chunyang, Zhou Tianen, Mo Mengxue, Lyu Wenquan, Gao Ming, Zhu Ruikai, Gao Zhiwei.
Action mechanism of metformin combined with Eomecon chionantha Hance dressing in treatment of deep second-degree burn wounds#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2001-2013.
|
| [4] | Lai Jiaming, , Song Yuling, Chen Zixi, Wei Jinghuan, Cai Hao, , Li Guoquan, . Screening of diagnostic markers for endothelial cell Senescence in mice with radiation-induced heart disease and analysis of immune infiltration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1450-1463. |
| [5] | Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591. |
| [6] | Hu Jing, Zhu Ling, Xie Juan, Kong Deying, Liu Doudou. Autophagy regulates early embryonic development in mice via affecting H3K4me3 modification [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1147-1155. |
| [7] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [8] | Dong Chao, Zhao Mohan, Liu Yunan, Yang Zeli, Chen Leqin, Wang Lanfang. Effects of magnetic nano-drug carriers on exercise-induced muscle injury and inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 345-353. |
| [9] | Wang Yaping, Gao Tianyun, Wang Bin. Senescence of human bone marrow mesenchymal stromal cells with increasing age is not dependent on the mediation of endogenous retroviruses [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 10-20. |
| [10] | Xue Hui, Li Dongnan, Zhao Yadi, Chen Chao, Xie Zongyuan. Relationship between BCR/ABL gene expression and recurrence before and after allogeneic transplantation in Ph chromosome positive acute lymphoblastic leukemia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 139-144. |
| [11] | Lyu Ruyue, Gu Lulu, Liu Qian, Zhou Siyi, Li Beibei, Xue Letian, Sun Peng. Regulatory mechanisms of exosome secretion and its application prospects in biomedicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 184-193. |
| [12] | Luo Wenbin, Li Ruoyun, Pan Chaofan, Luo Changjiang. Engineered exosomes for repairing tissue damage: application potential, excellent biological stability, and targeting specificity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 204-217. |
| [13] | Liu Ziwei, Nijati·Tursun, Yin Rui, Li Shuhui, Zhou Jing. Effect of cannabinoid type I receptors on neuronal differentiation of human apical papilla stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 93-100. |
| [14] | Xu Hao, Ding Lu, Li Xiao. Investigating the effect of the mechanical wear on abutment screw in Morse taper connection implant implant system by using finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [15] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||