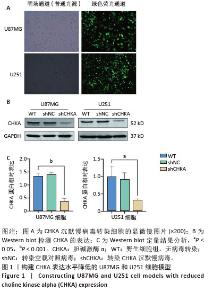
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 130-138.doi: 10.12307/2026.540
Previous Articles Next Articles
Choline kinase alpha silencing affects proliferation and apoptosis in glioma cells by inducing mitochondrial dysfunction
Zhao Yang1, Li Jialin2, Wu Xiao1, Zou Yourui3, Liu Yang3, Ma Hui3
- 1Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 2Tianjin Hospital, Tianjin 300000, China; 3General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China
-
Received:2024-11-18Accepted:2025-01-20Online:2026-01-08Published:2025-07-02 -
Contact:Ma Hui, PhD, Professor, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
About author:Zhao Yang, Master candidate, Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China -
Supported by:National Natural Science Foundation of China, No. 82460469 (to MH); Ningxia Natural Science Foundation, No. 2024AAC03591 (to LY); Ningxia Natural Science Foundation, No. 2022AAC03559 (to MH)
CLC Number:
Cite this article
Zhao Yang, Li Jialin, Wu Xiao, Zou Yourui, Liu Yang, Ma Hui. Choline kinase alpha silencing affects proliferation and apoptosis in glioma cells by inducing mitochondrial dysfunction[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 130-138.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

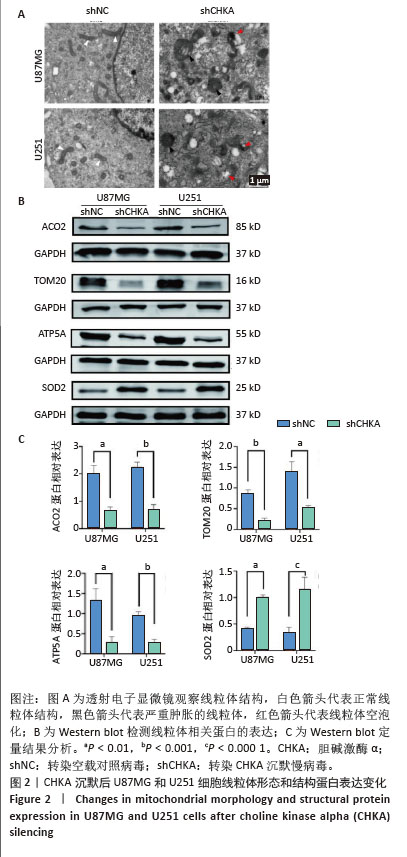
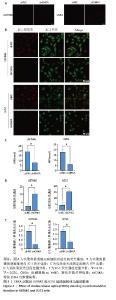
2.2 CHKA沉默对U87MG和U251细胞线粒体结构和形态的影响 透射电子显微镜观察结果显示,与shNC组U87MG和U251细胞比较,shCHKA组U87MG和U251细胞线粒体结构紊乱,出现线粒体肿胀、线粒体空泡化(图2A);Western blot实验结果显示(图2B,C),shCHKA组U87MG和U251细胞中ACO2、TOM20和ATP5A蛋白表达水平均较shNC组显著降低(P < 0.01,P < 0.001),提示CHKA沉默导致线粒体结构破坏和功能减弱[20-22];而SOD2蛋白表达水平显著升高(P < 0.01,P < 0.000 1),可能是细胞为应对线粒体功能障碍引发的活性氧积累而激活的代偿性抗氧化反应[23]。 "
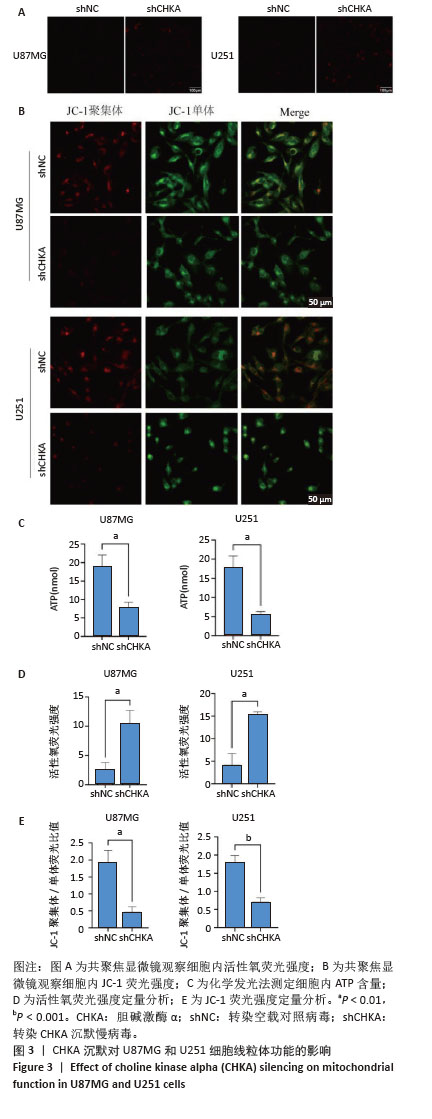
2.3 CHKA沉默对U87MG和U251细胞线粒体功能的影响 为了进一步明确CHKA沉默对线粒体功能的影响,分别检测了CHKA沉默前后U87MG和U251细胞活性氧、线粒体膜电位和细胞ATP含量的变化。利用荧光显微镜对各组细胞的活性氧水平进行检测,结果显示shCHKA组U87MG和U251细胞内活性氧荧光强度较shNC组显著升高(P < 0.01,图3A,D)。利用聚集体与单体的JC-1荧光比值来评估各组细胞的线粒体膜电位,荧光图像中红色荧光表示JC-1聚集体(高膜电位),绿色荧光表示JC-1单体(低膜电位),结果显示shCHKA 组JC-1聚集体/单体荧光比值较低,这表明CHKA沉默后U87MG和U251细胞的线粒体膜电位水平显著降低(P < 0.01,P < 0.001,图3B,E)。利用化学发光法检测各组细胞内ATP水平的变化,结果以纳米摩尔(nmol)表达,结果显示shCHKA组U87MG和U251细胞内ATP水平较shNC组显著降低(P < 0.01,图3C)。 "

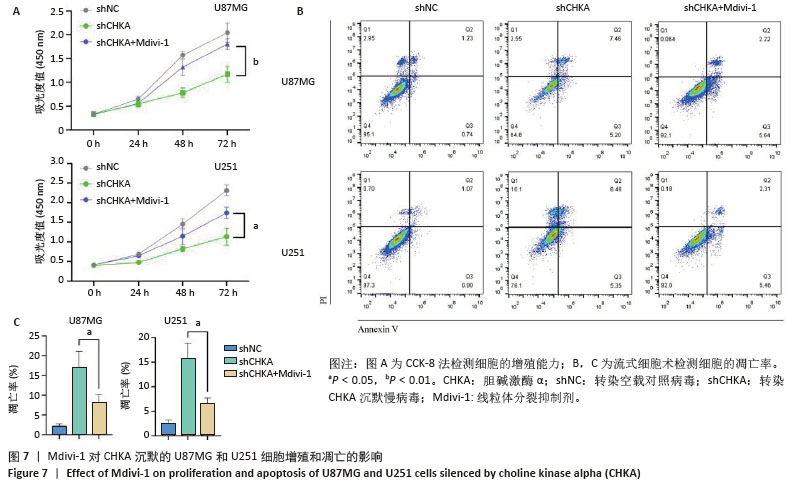
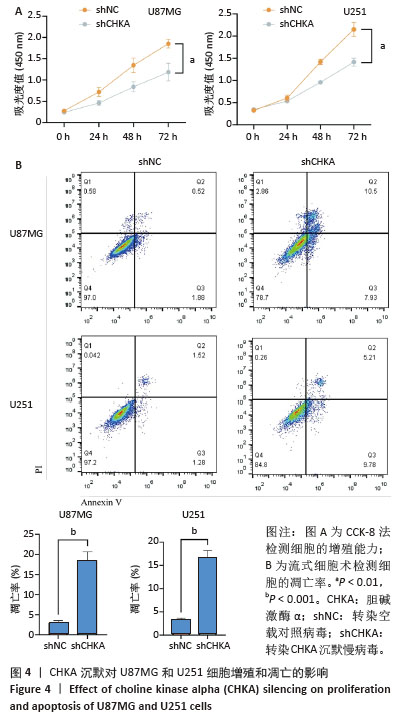
2.4 CHKA沉默对U87MG和U251细胞增殖和凋亡的影响 该研究验证了CHKA沉默会引起U87MG和U251细胞线粒体结构和功能的一系列损伤,同时活性氧增加等线粒体异常现象是细胞早期凋亡的标志事件之一[24-25],因此猜测CHKA沉默可以引起U87MG和U251细胞增殖能力和凋亡水平的变化。利用CCK-8实验检测各组U87MG和U251细胞的增殖能力,结果显示shCHKA组U87MG和U251细胞的增殖能力较shNC组显著减弱(P < 0.01,图4A)。利用流式细胞术检测各组U87MG和U251细胞的凋亡率,结果显示shCHKA组U87MG和U251细胞的凋亡率较shNC组显著升高(P < 0.001,图4B)。综上所述,通过在胶质瘤细胞中沉默CHKA,验证了CHKA沉默后胶质瘤细胞的线粒体发生了功能障碍,并可能进一步导致细胞增殖能力减弱和凋亡水平增加,影响了胶质瘤细胞的生长和恶性表型。 "
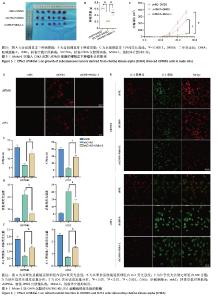
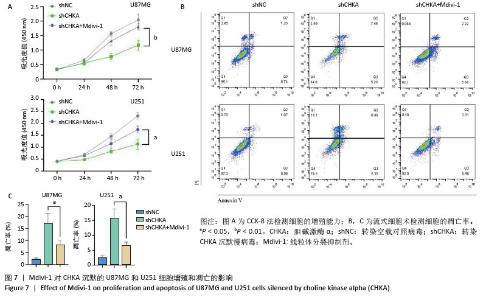
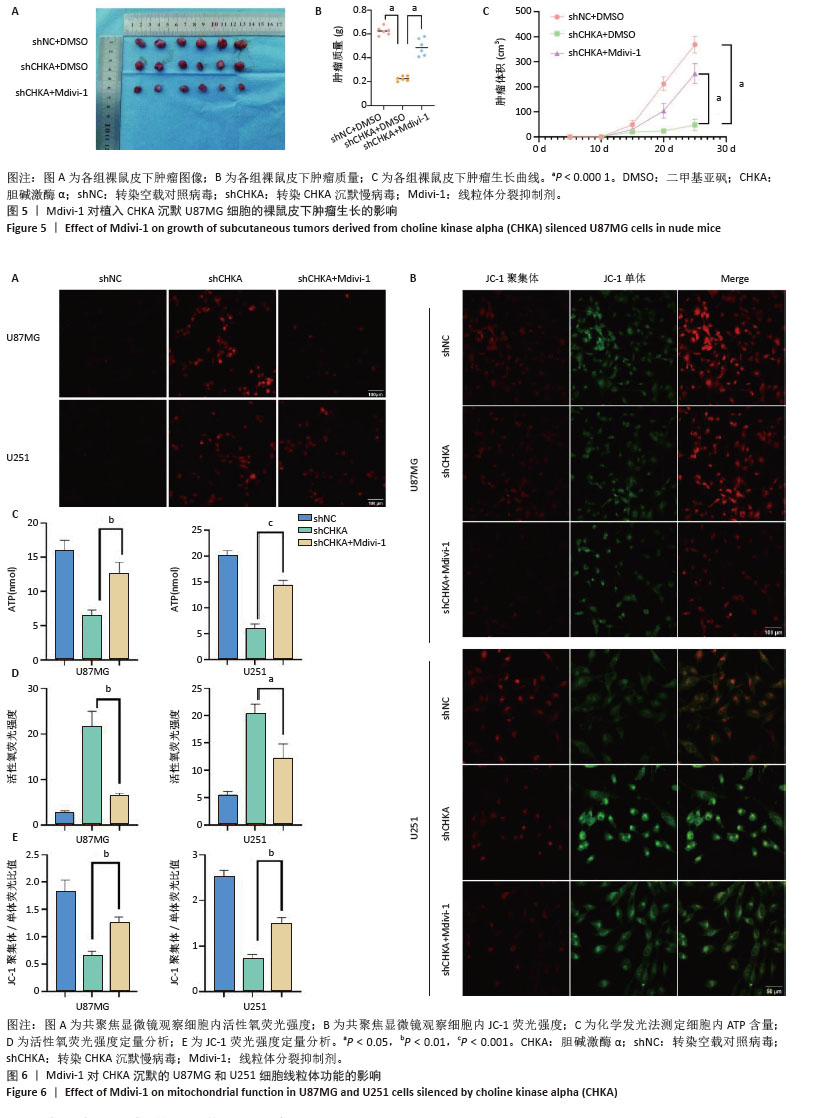
2.5 Mdivi-1对植入CHKA沉默U87MG细胞的裸鼠皮下肿瘤生长的影响 为体内体外共同验证以上结论,皮下植入U87MG胶质瘤细胞,记录肿瘤0-25 d体积变化,25 d切除肿瘤并记录肿瘤大小和肿瘤质量,结果显示:shCHKA组的裸鼠瘤体质量较shNC组明显减轻,生长速度显著降低(P < 0.000 1);同时为了明确CHKA沉默是通过改变线粒体功能从而导致胶质瘤的抑制作用,在CHKA沉默的基础上腹腔注射线粒体分裂抑制剂Mdivi-1,保护线粒体功能,反向验证CHKA沉默后对胶质瘤的抑制作用。结果显示:shCHKA+ Mdivi-1组的裸鼠瘤体质量较shCHKA组显著增加,生长速度显著变快(P < 0.000 1,图5)。 2.6 Mdivi-1对CHKA沉默的U87MG和U251细胞线粒体功能的影响 shCHKA+Mdivi-1组U87MG和U251细胞内活性氧荧光强度较shCHKA组显著降低(P < 0.05,P < 0.01,图6A,D)。shCHKA+Mdivi-1组U87MG和U251细胞线粒体膜电位较shCHKA组显著升高(P < 0.01,图6B,E)。shCHKA+Mdivi-1组U87MG和U251细胞内ATP水平较shCHKA组显著升高(P < 0.01,P < 0.001,图6C)。 "
| [1] TAN AC, ASHLEY DM, LÓPEZ GY, et al. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70(4): 299-312. [2] THOMAS AA, BRENNAN CW, DEANGELIS LM, et al. Emerging therapies for glioblastoma. JAMA Neurol. 2014;71(11):1437-1444. [3] LAN Z, LI X, ZHANG X. Glioblastoma: An Update in Pathology, Molecular Mechanisms and Biomarkers. Int J Mol Sci. 2024;25(5):3040. [4] MA Q, JIANG H, MA L, et al. The moonlighting function of glycolytic enzyme enolase-1 promotes choline phospholipid metabolism and tumor cell proliferation. Proc Natl Acad Sci U S A. 2023;120(15): e2209435120. [5] GOKHALE S, XIE P. ChoK-Full of Potential: Choline Kinase in B Cell and T Cell Malignancies. Pharmaceutics. 2021;13(6):911. [6] GLUNDE K, BHUJWALLA ZM. Choline kinase alpha in cancer prognosis and treatment. Lancet Oncol. 2007;8(10):855-857. [7] ZONG Y, LI H, LIAO P, et al. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):124. [8] GRANATH-PANELO M, KAJIMURA S. Mitochondrial heterogeneity and adaptations to cellular needs. Nat Cell Biol. 2024;26(5):674-686. [9] DECKER ST, FUNAI K. Mitochondrial membrane lipids in the regulation of bioenergetic flux. Cell Metab. 2024;36(9):1963-1978. [10] ZOU Y, HUANG L, SUN S, et al. Choline Kinase Alpha Promoted Glioma Development by Activating PI3K/AKT Signaling Pathway. Cancer Biother Radiopharm. 2021. doi: 10.1089/cbr.2021.0294. [11] HORVATH SE, DAUM G. Lipids of mitochondria. Prog Lipid Res. 2013; 52(4):590-614. [12] BEREITER-HAHN J, JENDRACH M. Mitochondrial dynamics. Int Rev Cell Mol Biol. 2010;284:1-65. [13] LEÃO BARROS MB, PINHEIRO DDR, BORGES BDN. Mitochondrial DNA Alterations in Glioblastoma (GBM). Int J Mol Sci. 2021;22(11):5855. [14] PENG Y, ZHAO T, RONG S, et al. Young small extracellular vesicles rejuvenate replicative senescence by remodeling Drp1 translocation-mediated mitochondrial dynamics. J Nanobiotechnology. 2024; 22(1):543. [15] PARK SW, KIM KY, LINDSEY JD, et al. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci. 2011;52(5):2837-2843. [16] ZHANG WK, YAN JM, CHU M, et al. Bunyavirus SFTSV nucleoprotein exploits TUFM-mediated mitophagy to impair antiviral innate immunity. Autophagy. 2025;21(1):102-119. [17] YANG Z, YANG Z, HU Z, et al. UAP1L1 plays an oncogene-like role in glioma through promoting proliferation and inhibiting apoptosis. Ann Transl Med. 2021;9(7):542. [18] 李艳花,张晓娟,张思羽,等.Mdivi-1通过抑制少突胶质细胞凋亡信号通路发挥髓鞘保护作用[J].中国病理生理杂志,2024,40(3): 527-534. [19] STRIBBLING SM, RYAN AJ. The cell-line-derived subcutaneous tumor model in preclinical cancer research. Nat Protoc. 2022;17(9):2108-2128. [20] PADALKO V, POSNIK F, ADAMCZYK M. Mitochondrial Aconitase and Its Contribution to the Pathogenesis of Neurodegenerative Diseases. Int J Mol Sci. 2024;25(18):9950. [21] PANDA PK, NAIK PP, MEHER BR, et al. PUMA dependent mitophagy by Abrus agglutinin contributes to apoptosis through ceramide generation. Biochim Biophys Acta Mol Cell Res. 2018;1865(3):480-495. [22] TANG D, KROEMER G, KANG R. Targeting cuproplasia and cuproptosis in cancer. Nat Rev Clin Oncol. 2024;21(5):370-388. [23] DING D, LI N, GE Y, et al. Current status of superoxide dismutase 2 on oral disease progression by supervision of ROS. Biomed Pharmacother. 2024;175:116605. [24] JENKINS BC, NEIKIRK K, KATTI P, et al. Mitochondria in disease: changes in shapes and dynamics. Trends Biochem Sci. 2024;49(4):346-360. [25] KENNY TC, BIRSOY K. Mitochondria and Cancer. Cold Spring Harb Perspect Med. 2024;14(12):a041534. [26] SUOMALAINEN A, NUNNARI J. Mitochondria at the crossroads of health and disease. Cell. 2024;187(11):2601-2627. [27] LI Y, ZHANG H, YU C, et al. New Insights into Mitochondria in Health and Diseases. Int J Mol Sci. 2024;25(18):9975. [28] ZHANG Y, YAN H, WEI Y, et al. Decoding mitochondria’s role in immunity and cancer therapy. Biochim Biophys Acta Rev Cancer. 2024; 1879(4):189107. [29] LIU BH, XU CZ, LIU Y, et al. Mitochondrial quality control in human health and disease. Mil Med Res. 2024;11(1):32. [30] ARLAUCKAS SP, POPOV AV, DELIKATNY EJ. Choline kinase alpha-Putting the ChoK-hold on tumor metabolism. Prog Lipid Res. 2016;63:28-40. [31] MIYAKE T, PARSONS SJ. Functional interactions between Choline kinase α, epidermal growth factor receptor and c-Src in breast cancer cell proliferation. Oncogene. 2012;31(11):1431-1441. [32] 黄灵,邹有瑞,马悦,等.胆碱激酶 α 在不同级别胶质瘤中的表达差异及临床意义[J].中国癌症杂志,2021,31(10):892-898. [33] 岳芳倩,邹有瑞,孙胜玉,等.敲低胆碱激酶α(CHKA)抑制U87MG人胶质瘤细胞增殖和侵袭及迁移[J].细胞与分子免疫学杂志, 2020,36(8):724-728. [34] 岳芳倩,邹有瑞,孙胜玉,等.胆碱激酶α抑制剂MN58b对U87胶质瘤干细胞增殖及凋亡的影响[J].中国免疫学杂志,2021, 37(12):1468-1471. [35] LI J, ZHAO Y, WU X, et al. Choline kinase alpha regulates autophagy-associated exosome release to promote glioma cell progression. Biochem Biophys Res Commun. 2025;746:151269. [36] RUBIO-RUIZ B, SERRÁN-AGUILERA L, HURTADO-GUERRERO R, et al. Recent advances in the design of choline kinase α inhibitors and the molecular basis of their inhibition. Med Res Rev. 2021;41(2):902-927. [37] CHEN X, QIU H, WANG C, et al. Molecular structure and differential function of choline kinases CHKα and CHKβ in musculoskeletal system and cancer. Cytokine Growth Factor Rev. 2017;33:65-72. [38] CASSIDY-STONE A, CHIPUK JE, INGERMAN E, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193-204. [39] SONG N, MEI S, WANG X, et al. Focusing on mitochondria in the brain: from biology to therapeutics. Transl Neurodegener. 2024;13(1):23. [40] RODRIGUES T, FERRAZ LS. Therapeutic potential of targeting mitochondrial dynamics in cancer. Biochem Pharmacol. 2020;182: 114282. |
| [1] | Wang Qisa, Lu Yuzheng, Han Xiufeng, Zhao Wenling, Shi Haitao, Xu Zhe. Cytocompatibility of 3D printed methyl acrylated hyaluronic acid/decellularized skin hydrogel scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1912-1920. |
| [2] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [3] | Liu Dawei, Cui Yingying, Wang Fanghui, Wang Zixuan, Chen Yuhan, Li Yourui, Zhang Ronghe. Epigallocatechin gallate-mediated bidirectional regulation of reactive oxygen species and its application in nanomaterials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2101-2112. |
| [4] | Sun Lei, Zhang Qi, Zhang Yu. Pro-osteoblastic effect of chlorogenic acid protein microsphere/polycaprolactone electrospinning membrane [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1877-1884. |
| [5] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [6] | Jia Jinwen, Airefate·Ainiwaer, Zhang Juan. Effects of EP300 on autophagy and apoptosis related to allergic rhinitis in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1439-1449. |
| [7] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [8] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [9] | Huang Liuyan, Zhang Wenxi, Chen Shuwen, Yu Shimei, Dai Zhong, Zuo Changqing. Forskolin promotes C2C12 myoblast differentiation via regulating the ERK and Akt signaling pathways [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1114-1121. |
| [10] | Hu Jing, Zhu Ling, Xie Juan, Kong Deying, Liu Doudou. Autophagy regulates early embryonic development in mice via affecting H3K4me3 modification [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1147-1155. |
| [11] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [12] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [13] | Bao Zhuoma, Hou Ziming, Jiang Lu, Li Weiyi, Zhang Zongxing, Liu Daozhong, Yuan Lin. Effect and mechanism by which Pterocarya hupehensis skan total flavonoids regulates the proliferation, migration and apoptosis of fibroblast-like synoviocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 816-823. |
| [14] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [15] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Mechanism by which vascular endothelial growth factor A targets regulation of angiogenesis in the treatment of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 671-679. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||