Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 101-110.doi: 10.12307/2025.567
Previous Articles Next Articles
Contribution and interaction of various cells in bone marrow microenvironment to exosomal circular RNA associated with multiple myeloma bone disease
Yu Manya1, Cui Xing2
- 1First School of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 2Cancer Center, Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250001, Shandong Province, China
-
Received:2024-09-20Accepted:2024-11-14Online:2026-01-08Published:2025-07-02 -
Contact:Cui Xing, MD, Chief physician, Professor, Doctoral supervisor, Cancer Center, Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250001, Shandong Province, China -
About author:Yu Manya, Master candidate, First School of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
Supported by:National Natural Science Foundation of China, No. 82074348, 82274491 (to CX); Shandong Natural Science Foundation Innovation and Development Joint Fund, No. ZR2023LZL009 (to CX)
CLC Number:
Cite this article
Yu Manya, Cui Xing. Contribution and interaction of various cells in bone marrow microenvironment to exosomal circular RNA associated with multiple myeloma bone disease[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 101-110.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
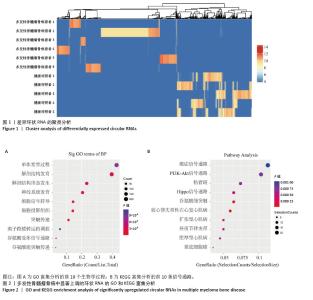
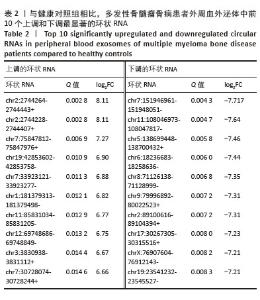
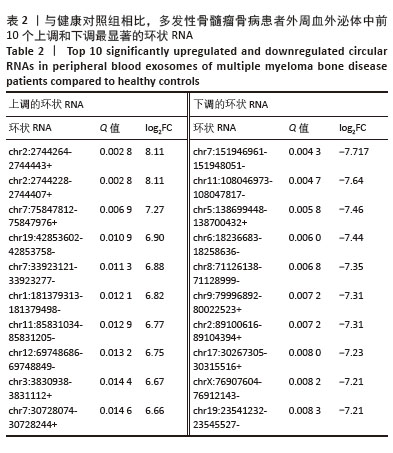
2.2 GO分析和KEGG分析 对多发性骨髓瘤骨病患者中表达水平显著上调的环状RNA进行GO和KEGG富集分析(图2A,B),结果显示差异环状RNA的亲本基因主要富集在神经系统发育、谷氨酸受体信号通路、细胞信号转导、癌症通路、PI3K-Akt信号通路、Hippo信号通路、致心律失常性右心室心肌病、扩张型心肌病、昼夜节律同步、肥厚型心肌病通路。相关研究表明,PI3K-Akt信号通路和Hippo信号通路与骨髓瘤细胞的存活、增殖、迁移和代谢密切相关[11-12]。此外,PI3K-Akt信号通路在骨重塑中发挥重要作用,抑制PI3K可以促进矿化,并抑制破骨细胞生成[13];Hippo信号通路可以与Wnt通路相互作用调节骨生成,其通路成分如YAP/TAZ/TEAD 复合物、RASSF2、MST2等也与破骨细胞成熟和功能有关,是多发性骨髓瘤骨病的重要调节因子[14-15]。 "
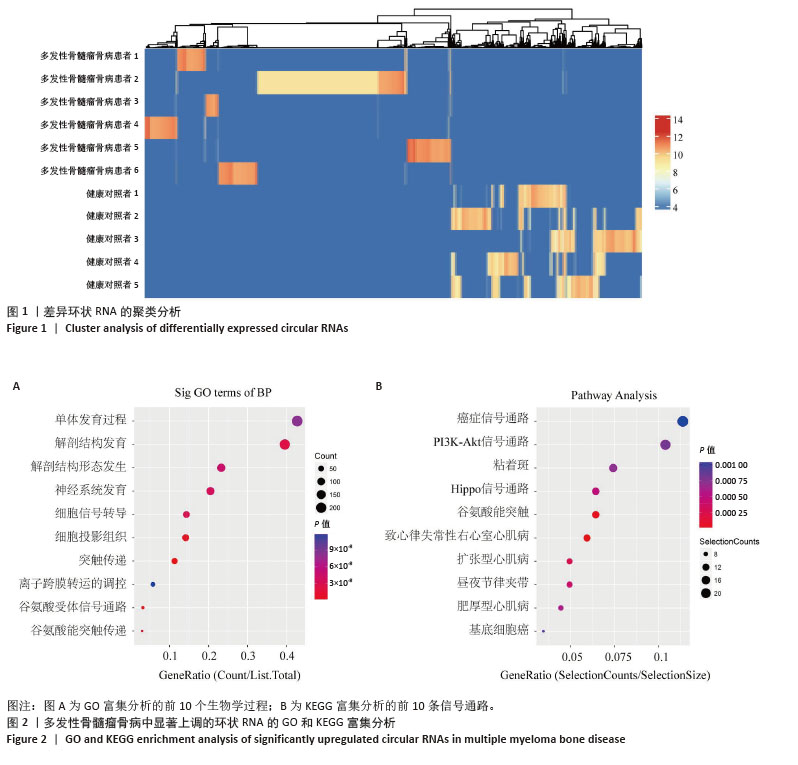

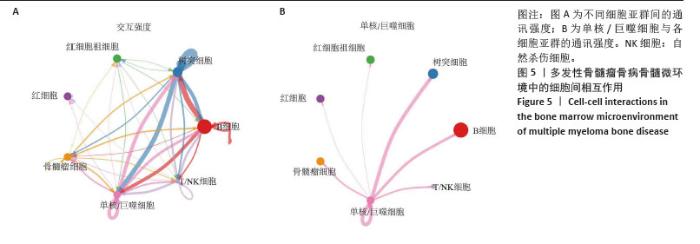
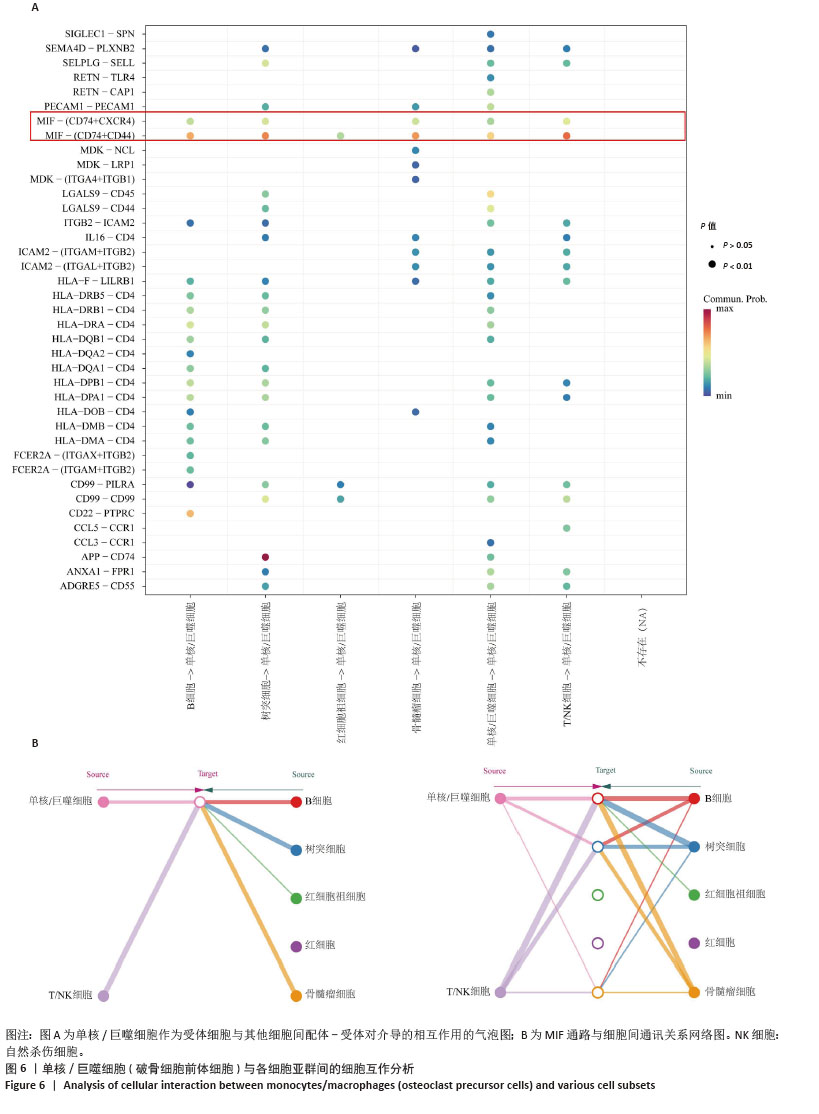
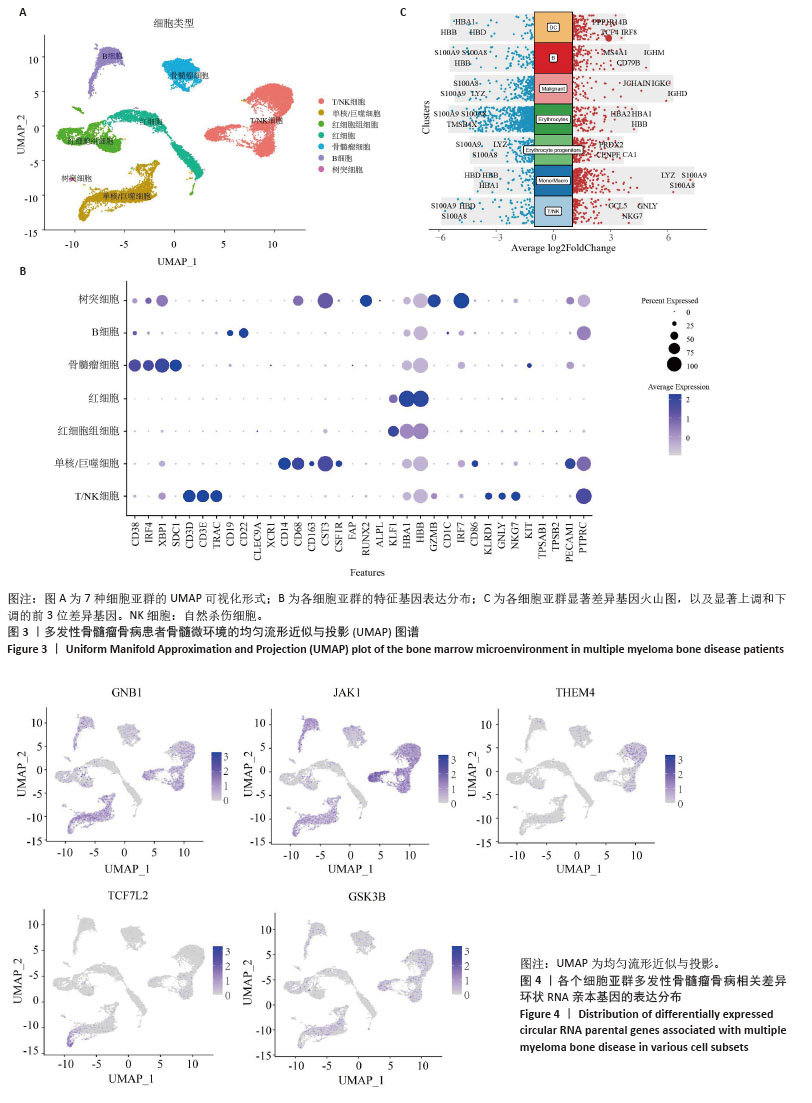
2.3 单细胞降维、聚类分析 为了推测与多发性骨髓瘤骨病相关的差异环状RNA的来源细胞,选取GSE271107数据集中4例多发性骨髓瘤患者的骨髓单细胞RNA测序数据。通过对单细胞RNA测序数据的质控、UMAP降维,获得了21 868个细胞的转录组数据。根据基因的表达对细胞进行自动注释和手动矫正,分别是T细胞/自然杀伤细胞(7 351个)、单核细胞/巨噬细胞(4 077个)、红细胞祖细胞(2 849个)、红细胞(2 585个)、骨髓瘤细胞(2 508个)、B细胞(2 380个)、树突状细胞(118个),见图3A,B。通过对7个注释出的细胞亚群进行差异化分析,并绘制火山图,可以看出在不同细胞亚群中显著上调和下调的前3位差异基因,见图3C。 2.4 外泌体环状RNA溯源 将多发性骨髓瘤骨病相关差异环状RNA的亲本基因(参与PI3K-Akt通路和Hippo通路的差异基因)通过单细胞RNA测序的聚类特征图(图4)可视化,发现GNB1、JAK1、THEM4、TCF7L2、GSK3B分布在特定的细胞亚群中,GNB1和JAK1主要分布在T细胞/自然杀伤细胞、B细胞和单核/巨噬细胞中,GSK3B主要分布在T细胞/自然杀伤细胞和单核/巨噬细胞中,TCF7L2主要分布在单核/巨噬细胞中,THEM4主要分布在T细胞/自然杀伤细胞中,由此推测多发性骨髓瘤骨病相关差异环状RNA主要来源于骨髓微环境中的T细胞/自然杀伤细胞、B细胞和单核细胞/巨噬细胞。 "
| [1] MALARD F, NERI P, BAHLIS NJ, et al. Multiple myeloma. Nat Rev Dis Primers. 2024;10(1):45. [2] MUKKAMALLA SKR, MALIPEDDI D. Myeloma Bone Disease: A Comprehensive Review. Int J Mol Sci. 2021;22(12):6208. [3] YU D, LI Y, WANG M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56. [4] BERNSTEIN ZS, KIM EB, RAJE N. Bone Disease in Multiple Myeloma: Biologic and Clinical Implications. Cells. 2022;11(15): 2308. [5] MENU E, VANDERKERKEN K. Exosomes in multiple myeloma: from bench to bedside. Blood. 2022;140(23):2429-2442. [6] LIU R, ZHONG Y, CHEN R, et al. m6A reader hnRNPA2B1 drives multiple myeloma osteolytic bone disease. Theranostics. 2022; 12(18):7760-7774. [7] LI B, XU H, HAN H, et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene. 2018;37(41): 5508-5519. [8] UNTI MJ, JAFFREY SR. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem Biol. 2024; 31(1):163-176.e5. [9] ZHANG F, JIANG J, QIAN H, et al. Exosomal circRNA: emerging insights into cancer progression and clinical application potential. J Hematol Oncol. 2023;16(1):67. [10] ARAN D, LOONEY AP, LIU L, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20(2):163-172. [11] KIKUCHI H, AMOFA E, MCENERY M, et al. Inhibition of PI3K Class IA Kinases Using GDC-0941 Overcomes Cytoprotection of Multiple Myeloma Cells in the Osteoclastic Bone Marrow Microenvironment Enhancing the Efficacy of Current Clinical Therapeutics. Cancers (Basel). 2023;15(2):462. [12] LI H, ZHANG Y, MOU X, et al. Interference with PLA2G16 promotes cell cycle arrest and apoptosis and inhibits the reprogramming of glucose metabolism in multiple myeloma cells by modulating the Hippo/YAP signaling pathway. Anticancer Drugs. 2024;35(10):902-911. [13] TSUBAKI M, KATO C, MANNO M, et al. Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem. 2007;304(1-2):53-60. [14] PAN JX, XIONG L, ZHAO K, et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Res. 2018;6:18. [15] YANG W, HAN W, QIN A, et al. The emerging role of Hippo signaling pathway in regulating osteoclast formation. J Cell Physiol. 2018;233(6): 4606-4617. [16] MIAOMIAO S, XIAOQIAN W, YUWEI S, et al. Cancer-associated fibroblast-derived exosome microRNA-21 promotes angiogenesis in multiple myeloma. Sci Rep. 2023;13(1):9671. [17] MIZUHARA K, SHIMURA Y, TSUKAMOTO T, et al. Tumour-derived exosomes promote the induction of monocytic myeloid-derived suppressor cells from peripheral blood mononuclear cells by delivering miR-106a-5p and miR-146a-5p in multiple myeloma. Br J Haematol. 2023;203(3):426-438. [18] WANG Z, HE J, BACH DH, et al. Induction of m6A methylation in adipocyte exosomal LncRNAs mediates myeloma drug resistance. J Exp Clin Cancer Res. 2022;41(1):4. [19] YU M, JI L, LI S, et al. Exosomal circ-CACNG2 promotes cardiomyocyte apoptosis in multiple myeloma via modulating miR-197-3p/caspase3 axis. Exp Cell Res. 2022;417(2):113229. [20] ALIMOHAMMADI M, RAHIMZADEH P, KHORRAMI R, et al. A comprehensive review of the PTEN/PI3K/Akt axis in multiple myeloma: From molecular interactions to potential therapeutic targets. Pathol Res Pract. 2024;260:155401. [21] HEINEMANN L, MÖLLERS KM, AHMED HMM, et al. Inhibiting PI3K-AKT-mTOR Signaling in Multiple Myeloma-Associated Mesenchymal Stem Cells Impedes the Proliferation of Multiple Myeloma Cells. Front Oncol. 2022;12:874325. [22] KYRIAZOGLOU A, NTANASIS-STATHOPOULOS I, TERPOS E, et al. Emerging Insights Into the Role of the Hippo Pathway in Multiple Myeloma and Associated Bone Disease. Clin Lymphoma Myeloma Leuk. 2020;20(2):57-62. [23] YANG J, ZHANG X, WANG J, et al. Anti beta2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts. Blood. 2007;110(8):3028-3035. [24] MENG B, WU D, CHENG Y, et al. Interleukin-20 differentially regulates bone mesenchymal stem cell activities in RANKL-induced osteoclastogenesis through the OPG/RANKL/RANK axis and the NF-κB, MAPK and AKT signalling pathways. Scand J Immunol. 2020;91(5): e12874. [25] ZHANG Z, ZHANG X, ZHAO D, et al. TGF‑β1 promotes the osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1 signalling pathway. Mol Med Rep. 2019;19(5):3505-3518. [26] MARTIN SK, GAN ZY, FITTER S, et al. The effect of the PI3K inhibitor BKM120 on tumour growth and osteolytic bone disease in multiple myeloma. Leuk Res. 2015;39(3):380-387. [27] SEO E, BASU-ROY U, GUNARATNE PH, et al. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3(6):2075-2087. [28] MATSUMOTO Y, LA ROSE J, KENT OA, et al. Reciprocal stabilization of ABL and TAZ regulates osteoblastogenesis through transcription factor RUNX2. J Clin Invest. 2016;126(12):4482-4496. [29] PARK H, NOH AL, KANG JH, et al. Peroxiredoxin II negatively regulates lipopolysaccharide-induced osteoclast formation and bone loss via JNK and STAT3. Antioxid Redox Signal. 2015;22(1):63-77. [30] LU J, YE C, HUANG Y, et al. Corilagin suppresses RANKL-induced osteoclastogenesis and inhibits oestrogen deficiency-induced bone loss via the NF-κB and PI3K/AKT signalling pathways. J Cell Mol Med. 2020;24(18):10444-10457. [31] HUANG F, WONG P, LI J, et al. Osteoimmunology: The correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J Cell Mol Med. 2022;26(13):3591-3597. [32] WU LZ, DUAN DM, LIU YF, et al. Nicotine favors osteoclastogenesis in human periodontal ligament cells co-cultured with CD4(+) T cells by upregulating IL-1β. Int J Mol Med. 2013;31(4):938-942. [33] NAGAI S, KUREBAYASHI Y, KOYASU S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann N Y Acad Sci. 2013;1280: 30-34. [34] FU M, HU Y, LAN T, et al. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. 2022;7(1):376. [35] SÖDERSTRÖM K, STEIN E, COLMENERO P, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci U S A. 2010;107(29):13028-13033. [36] FENG P, LUO L, YANG Q, et al. Hippo kinases Mst1 and Mst2 maintain NK cell homeostasis by orchestrating metabolic state and transcriptional activity. Cell Death Dis. 2024;15(6):430. [37] ALI AK, NANDAGOPAL N, LEE SH. IL-15-PI3K-AKT-mTOR: A Critical Pathway in the Life Journey of Natural Killer Cells. Front Immunol. 2015;6:355. [38] CHEN Y, WANG H, NI Q, et al. B-Cell-Derived TGF-β1 Inhibits Osteogenesis and Contributes to Bone Loss in Periodontitis. J Dent Res. 2023;102(7):767-776. [39] FISCHER V, HAFFNER-LUNTZER M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14-21. [40] GRČEVIĆ D, SANJAY A, LORENZO J. Interactions of B-lymphocytes and bone cells in health and disease. Bone. 2023;168:116296. [41] HODSON DJ, TURNER M. The role of PI3K signalling in the B cell response to antigen. Adv Exp Med Biol. 2009;633:43-53. [42] ZHENG L, GAO J, JIN K, et al. Macrophage migration inhibitory factor (MIF) inhibitor 4-IPP suppresses osteoclast formation and promotes osteoblast differentiation through the inhibition of the NF-κB signaling pathway. FASEB J. 2019;33(6):7667-7683. [43] HE J, ZHENG L, LI X, et al. Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss. J Adv Res. 2023;53:235-248. |
| [1] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [2] | Xu Jiamu, Yang Cheng, Li Weimin, Wang Chunqing. Role and pathogenesis of pyroptosis and inflammatory factors in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 691-700. |
| [3] | Zuo Na, Tang Qi, Yu Meng, Tao Kai. Effect of miR-196b-5p in adipose-derived stem cell exosomes on burn wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 43-49. |
| [4] | Yuan Weiyuan, Lei Qinhui, Li Xiuqi, Lu Tiezhu, Fu Ziwen, Liang Zhili, Ji Shaoyang, Li Yijia, Ren Yu . Therapeutic effects of adipose-derived mesenchymal stem cells and their exosomes on dexamethasone-induced sarcopenia in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 58-67. |
| [5] | Zhang Tingting, Li Yalong, Yue Haodi, Li Yanjun, Geng Xiwen, Zhang Yuwei, Liu Xiaozhuan. Protection of exosomes derived from bone marrow mesenchymal stem cells of different mouse ages on radiation-induced lung injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 1-9. |
| [6] | Zhang Zhaowei, Chen Ouzile, Bai Mingru, Wang Chenglin. Therapeutic potential of bioactive substances secreted by dental mesenchymal stem cells for bone repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 163-174. |
| [7] | Liu Nian, Dong Xinyue, Wang Songpeng, Xu Yingjiang, Zhang Xiaoming. Stem cell exosomes and biomaterial-assisted exosomes in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 175-183. |
| [8] | Lyu Ruyue, Gu Lulu, Liu Qian, Zhou Siyi, Li Beibei, Xue Letian, Sun Peng. Regulatory mechanisms of exosome secretion and its application prospects in biomedicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 184-193. |
| [9] | Liu Yu, Gong Senyi, Yang Lihua, Li Weifeng, Hu Yuwen, Yan Qinbiao, Guo Meijin. Isolation, identification, and application of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 194-203. |
| [10] | Luo Wenbin, Li Ruoyun, Pan Chaofan, Luo Changjiang. Engineered exosomes for repairing tissue damage: application potential, excellent biological stability, and targeting specificity [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 204-217. |
| [11] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [12] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [13] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [14] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [15] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||