Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (30): 6398-6408.doi: 10.12307/2025.766
Previous Articles Next Articles
Metformin inhibits ferroptosis and improves cartilage damage in osteoarthritis model rats
Fan Jiaxin1, 2, Jia Xiang1, 2, Xu Tianjie1, 2, Liu Kainan3, Guo Xiaoling1, 2, Zhang Hui4, Wang Qian1, 2
- 1School of Basic Medical Sciences, North China University of Science and Technology, Tangshan 063210, Hebei Province, China; 2Key Laboratory of Basic Medicine for Chronic Diseases, Tangshan 063210, Hebei Province, China; 3College of Basic Medicine, Xingtai Medical College, Xingtai 054000, Hebei Province, China; 4First Department of Joint Surgery, Tangshan Second Hospital, Tangshan 063000, Hebei Province, China
-
Received:2024-08-10Accepted:2024-09-29Online:2025-10-28Published:2025-03-27 -
Contact:Wang Qian, MD, Master’s supervisor, School of Basic Medical Sciences, North China University of Science and Technology, Tangshan 063210, Hebei Province, China; Key Laboratory of Basic Medicine for Chronic Diseases, Tangshan 063210, Hebei Province, China -
About author:Fan Jiaxin, Master candidate, School of Basic Medical Sciences, North China University of Science and Technology, Tangshan 063210, Hebei Province, China; Key Laboratory of Basic Medicine for Chronic Diseases, Tangshan 063210, Hebei Province, China -
Supported by:Three-Three Talent Project, No. C20221117 (to ZH); Hebei Province 2023 Medical Science Research Project, No. 20230215 (to WQ)
CLC Number:
Cite this article
Fan Jiaxin, Jia Xiang, Xu Tianjie, Liu Kainan, Guo Xiaoling, Zhang Hui, Wang Qian . Metformin inhibits ferroptosis and improves cartilage damage in osteoarthritis model rats[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6398-6408.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
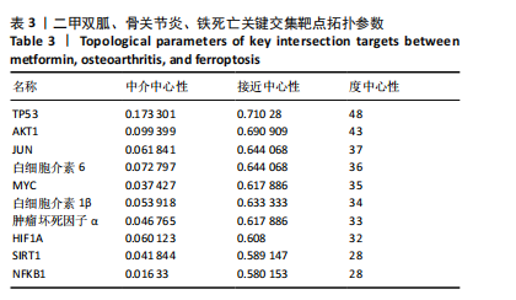
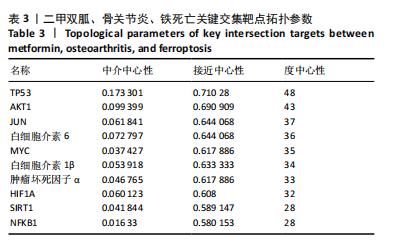
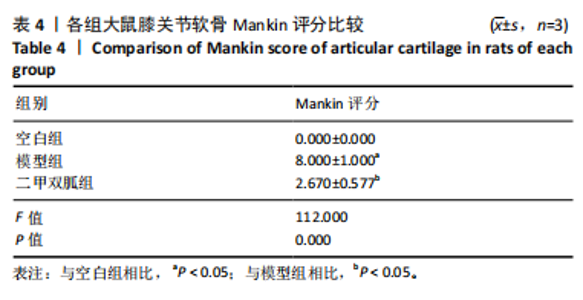
2.1 网络药理学结果 2.1.1 共同靶点预测 从CTD数据库中获得921个二甲双胍靶点,从SwissTarget数据库中收集了33个二甲双胍靶点,去除重复目标后,收集到949个二甲双胍靶点。从 GeneCards 数据库中总共获得 5 407 个骨关节炎相关靶点,从OMIM数据库中收集381个骨关节炎靶点,去除重复目标后,收集到5 624个骨关节炎靶点。从GeneCards数据库中总共获得1 467个铁死亡相关靶点,从OMIM数据库中收集54个铁死亡靶点,去除重复目标后,收集到1 505个铁死亡靶点。将3组靶点进行交叉,最终获得二甲双胍、骨关节炎和铁死亡的96个潜在共同靶点,见图1。 2.1.2 蛋白质-蛋白质相互作用网络分析 将获得的 96个二甲双胍、骨关节炎和铁死亡的潜在共同靶点转移至STRING平台进行蛋白质-蛋白质相互作用网络分析,并在 Cytoscape 3.9.1软件中进行可视化,最后构建了二甲双胍、骨关节炎和铁死亡的相互作用网络。节点的大小和颜色基于度值,而边缘厚度和颜色基于组合分数的大小,共有77个节点(代表77个潜在共同目标)和507条边(代表77个潜在共同目标之间的交互),见图2。 2.1.3 关键靶标 根据网络拓扑的中心性,数据包括度中心性、接近中心性和介数中心性。筛选条件是度值> 28,介数中心性> 0.005,接近中心性> 0.5。筛选出10个靶点,表明这 10个靶点可能是二甲双胍、骨关节炎和铁死亡的潜在关键靶点。按度值排名前10位是TP53、AKT1、JUN、白细胞介素6、MYC、白细胞介素1β、肿瘤坏死因子α、HIF1A、SIRT1、NFKB1,见表3。"
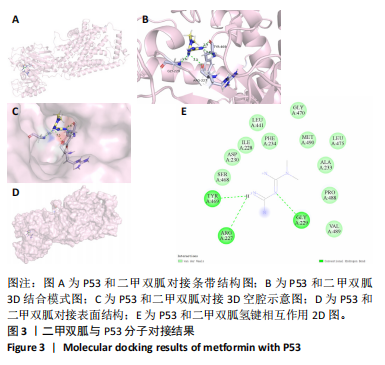
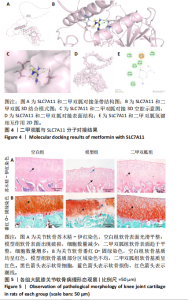
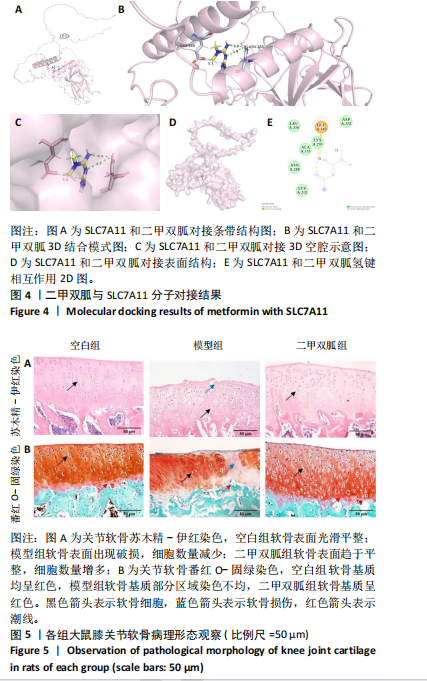
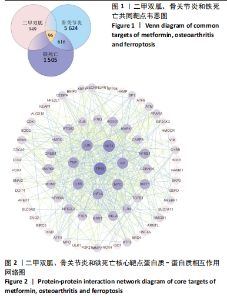
2.2 P53/SLC7A11与二甲双胍分子对接验证 使用软件Discovery Studio 2019进行分子对接模拟,以确定二甲双胍和P53/SLC7A11之间是否存在直接联系。对接分析结果显示,二甲双胍与P53/SLC7A11结合牢固稳定。可视化(使用pymol)显示:P53的活性氨基酸残基(ASN288和GLU349)直接与二甲双胍相互作用;在P53复合物的晶体结构中,二甲双胍与ASN288分别在0.33,0.39 nm处形成氢键相互作用,二甲双胍与GLU349在0.53 nm处形成盐桥作用,见图3。SLC7A11的活性氨基酸残基(ARG227、GLY229和TRY469)直接与二甲双胍相互作用;在Slc7a11复合物的晶体结构中,二甲双胍与ARG227、GLY229、TRY469分别在0.35,0.36,0.29,0.34 nm处形成氢键相互作用,见图4。 2.3 实验动物数量分析 30只SD大鼠全部进入结果分析。 2.4 各组大鼠膝关节软骨病理形态 苏木精-伊红染色结果:空白组大鼠软骨表面光滑,组织形态正常,软骨细胞单个排列;模型组大鼠软骨表面不规则,可见较明显缺损,软骨细胞呈簇状分布且数量减少;二甲双胍组软骨表面光滑,软骨细胞数量较多且排列整齐,见图5A。 番红O-固绿染色结果:空白组软骨细胞内可见蓝色细胞核,软骨基质番红染色均匀,呈红色,潮线完整;模型组软骨细胞数量减少,部分软骨组织染色不均匀,且潮线不完整;二甲双胍组软骨细胞数量较多,软骨组织染色较模型组染色均匀且深染,潮线完整,见图5B。 Mankin评分结果:模型组Mankin评分较空白组明显升高(P < 0.05),二甲双胍组Mankin评分较模型组明显降低(P < 0.05),见表4。"

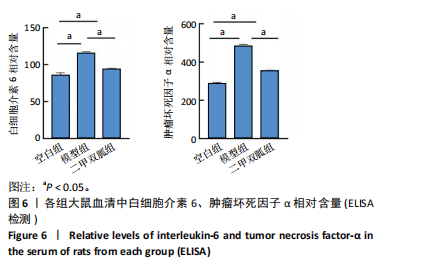
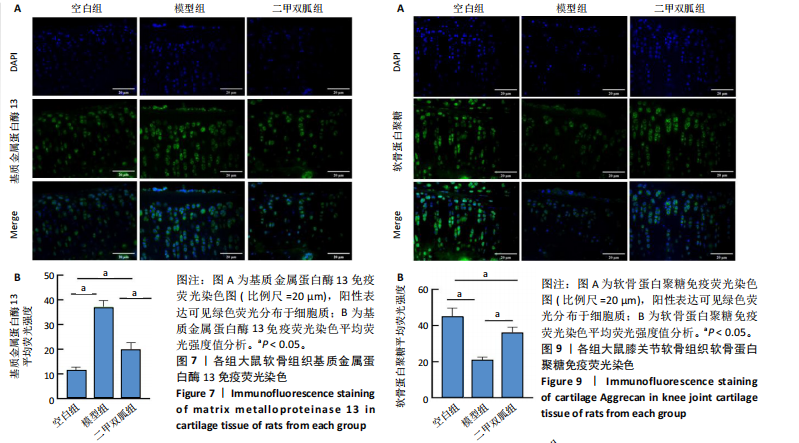
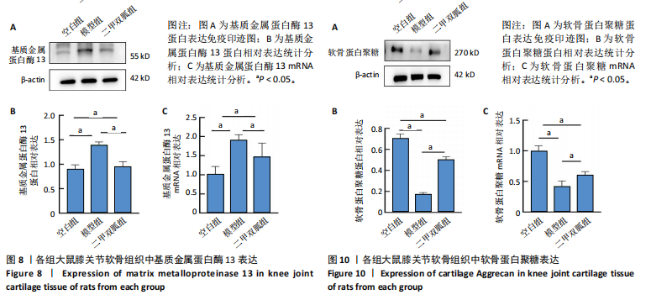
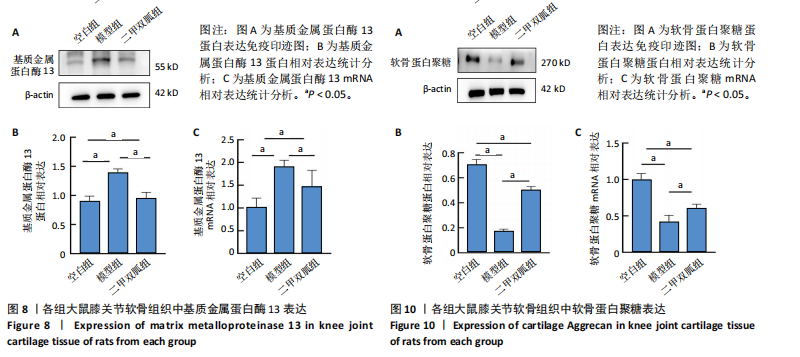
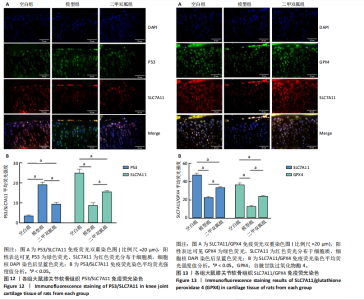
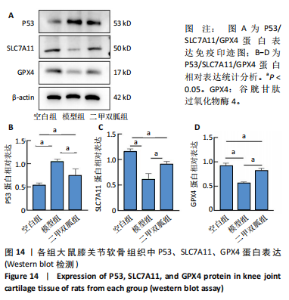
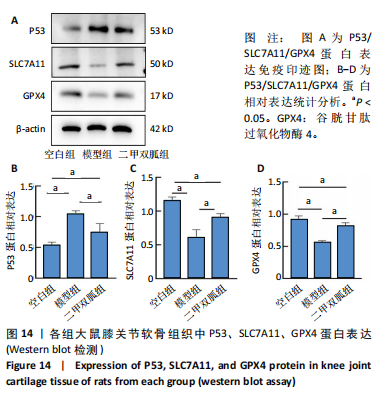
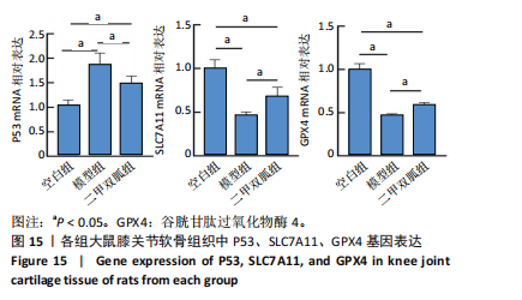
2.5 各组大鼠血清中白细胞介素6、肿瘤坏死因子α相对含量 模型组大鼠血清中白细胞介素6、肿瘤坏死因子α相对含量明显高于空白组(P < 0.05);二甲双胍组大鼠血清中白细胞介素6、肿瘤坏死因子α相对含量低于模型组(P < 0.05),见图6。 2.6 各组大鼠膝关节软骨组织中基质金属蛋白酶13表达 正置荧光显微镜观察,各组软骨细胞均可见基质金属蛋白酶13阳性反应呈绿色荧光,细胞核DAPI染色后呈蓝色荧光。空白组绿色荧光较弱,模型组荧光较空白组明显增强,二甲双胍组荧光较模型组明显减弱,见图7A。通过免疫荧光染色平均荧光强度分析发现,与空白组相较,模型组基质金属蛋白酶13平均荧光强度明显升高(P < 0.05);二甲双胍组较模型组平均荧光强度明显下降(P < 0.05),见图7B。 Western blot检测结果显示,模型组基质金属蛋白酶13蛋白表达最高,二甲双胍组次之,空白组最低,见图8A。对蛋白条带灰度值分析发现,模型组基质金属蛋白酶13蛋白表达较空白组明显升高(P < 0.05),二甲双胍组较模型组明显下降但仍高于空白组,差异有显著性意义(P < 0.05),见图8B。 Real-time qPCR检测结果显示,模型组基质金属蛋白酶13 mRNA相对表达量较空白组明显升高(P < 0.05),二甲双胍组较模型组明显降低但仍高于空白组,差异有显著性意义(P < 0.05),见图8C。Real-time qPCR结果与免疫荧光染色、Western blot检测结果一致。 2.7 各组大鼠膝关节软骨组织中软骨蛋白聚糖表达 正置荧光显微镜观察,各组软骨细胞均可见软骨蛋白聚糖阳性反应呈绿色荧光,细胞核DAPI染色后呈蓝色荧光。空白组绿色荧光较强,模型组荧光较空白组明显减弱,二甲双胍组荧光较模型组增强,见图9A。通过免疫荧光染色平均荧光强度分析发现,与空白组比较,模型组软骨蛋白聚糖荧光强度明显下降(P < 0.05);二甲双胍组较模型组荧光强度明显升高(P < 0.05),见图9B。 Western blot检测结果显示,模型组软骨蛋白聚糖蛋白表达最低,二甲双胍组次之,空白组最高,见图10A。对蛋白条带灰度值分析发现,模型组软骨蛋白聚糖蛋白表达较空白组明显下降(P < 0.05),二甲双胍组较模型组明显升高但仍低于空白组(P < 0.05),见图10B。 Real-time qPCR检测结果显示,模型组软骨蛋白聚糖mRNA相对表达量较空白组明显下降(P < 0.05),二甲双胍组较模型组明显升高(P < 0.05),见图10C。Real-time qPCR结果与免疫荧光染色、Western blot检测结果一致。 2.8 各组大鼠血清中谷胱甘肽、丙二醛、Fe2+相对含量 与空白组比较,模型组血清中丙二醛、Fe2+相对含量明显升高,谷胱甘肽相对含量明显降低(P < 0.05);与模型组比较,二甲双胍组血清中丙二醛、Fe2+相对含量明显降低,谷胱甘肽相对含量明显升高(P < 0.05),见图11。 2.9 各组大鼠膝关节软骨组织中P53/SLC7A11/GPX4表达 正置荧光显微镜观察,各组软骨细胞均可见P53阳性反应呈绿色荧光,SLC7A11阳性反应呈红色荧光,细胞核DAPI染色后呈蓝色荧光。P53荧光染色可见,空白组绿色荧光较弱,模型组荧光较空白组明显增强,二甲双胍组荧光较模型组明显减弱。同时,SLC7A11荧光染色可见,空白组红色荧光较强,模型组荧光较空白组明显减弱,二甲双胍组荧光较模型组明显增强,见图12A。免疫荧光染色平均荧光强度分析发现,与空白组相较,模型组P53荧光强度明显升高(P < 0.05);二甲双胍组较模型组荧光强度明显下降(P < 0.05)。同时,与空白组相较,模型组SLC7A11荧光强度明显下降(P < 0.05);二甲双胍组较模型组荧光强度明显升高(P < 0.05),见图12B。 正置荧光显微镜观察,各组软骨细胞均可见SLC7A11阳性反应呈红色荧光,GPX4阳性反应呈绿色荧光,细胞核DAPI染色后呈蓝色荧光。SLC7A11、GPX4荧光染色可见,空白组红、绿色荧光均较强;模型组荧光较空白组均明显减弱;二甲双胍组荧光较模型组明显增强,见图13A。"
| [1] TONG L, YU H, HUANG X, et al. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022;10(1):60. [2] HUNTER DJ, BIERMA-ZEINSTRA S. Osteoarthritis. Lancet. 2019;393(10182):1745-1759. [3] WANG H, SU J, YU M, et al. PGC-1α in osteoarthritic chondrocytes: From mechanism to target of action. Front Pharmacol. 2023;14:1169019. [4] KUSWANTO W, BAKER MC. Repurposing drugs for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2024;32(8):886-895. [5] ARODA VR, KNOWLER WC, CRANDALL JP, et al. Metformin for diabetes prevention: insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia. 2017;60(9):1601-1611. [6] WANG C, YANG Y, ZHANG Y, et al. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci Trends. 2019;12(6):605-612. [7] TERKELTAUB R, YANG B, LOTZ M, et al. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum. 2011;63(7):1928-1937. [8] YAO X, SUN K, YU S, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Translat. 2020;27:33-43. [9] XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369-379. [10] ZOU Z, HU W, KANG F, et al. Interplay between lipid dysregulation and ferroptosis in chondrocytes and the targeted therapy effect of metformin on osteoarthritis. J Adv Res. 2024. doi: 10.1016/j.jare.2024.04.012. [11] YI Y, ZHANG W, YI J, et al. Role of p53 Family Proteins in Metformin Anti-Cancer Activities. J Cancer. 2019;10(11):2434-2442. [12] LIU Y, GU W. p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ. 2022;29(5):895-910. [13] JIANG L, KON N, LI T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57-62. [14] KANG R, KROEMER G, TANG D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162-168. [15] YAN J, FENG G, MA L, et al. Metformin alleviates osteoarthritis in mice by inhibiting chondrocyte ferroptosis and improving subchondral osteosclerosis and angiogenesis. J Orthop Surg Res. 2022;17(1):333. [16] LI J, ZHANG B, LIU WX, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis. 2020;79(5):635-645. [17] 罗臻,李宏栩,卢启贵,等.红姜提取物保护早期膝骨关节炎模型大鼠的关节软骨[J].中国组织工程研究,2021,25(32):5155-5161. [18] 徐田杰,樊佳欣,郭小玲,等.二甲双胍抑制PI3K/AKT/mTOR信号通路保护骨关节炎模型大鼠关节软骨[J].中国组织工程研究,2025,29(5):1003-1012. [19] GEZER HH, OSTOR A. What is new in pharmacological treatment for osteoarthritis? Best Pract Res Clin Rheumatol. 2023;37(2):101841. [20] PERRUCCIO AV, YOUNG JJ, WILFONG JM, et al. Osteoarthritis year in review 2023: Epidemiology & therapy. Osteoarthritis Cartilage. 2024;32(2):159-165. [21] FLORY J, LIPSKA K. Metformin in 2019. JAMA. 2019;321(19):1926-1927. [22] TRIGGLE CR, MOHAMMED I, BSHESH K, et al. Metformin: Is it a drug for all reasons and diseases? Metabolism. 2022;133:155223. [23] LV Z, GUO Y. Metformin and Its Benefits for Various Diseases. Front Endocrinol (Lausanne). 2020;11:191. [24] LI D, RUAN G, ZHANG Y, et al. Metformin attenuates osteoarthritis by targeting chondrocytes, synovial macrophages and adipocytes. Rheumatology (Oxford). 2023;62(4):1652-1661. [25] NA HS, KWON JY, LEE SY, et al. Metformin Attenuates Monosodium-Iodoacetate-Induced Osteoarthritis via Regulation of Pain Mediators and the Autophagy-Lysosomal Pathway. Cells. 2021;10(3):681. [26] LAI FTT, YIP BHK, HUNTER DJ, et al. Metformin use and the risk of total knee replacement among diabetic patients: a propensity-score-matched retrospective cohort study. Sci Rep. 2022;12(1):11571. [27] FENG X, PAN J, LI J, et al. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging (Albany NY). 2020;12(2):1087-1103. [28] FUJII Y, LIU L, YAGASAKI L, et al. Cartilage Homeostasis and Osteoarthritis. Int J Mol Sci. 2022;23(11):6316. [29] GAO Y, LIU S, HUANG J, et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459. [30] HOLLANDER AP, DICKINSON SC, KAFIENAH W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cells. 2010;28(11):1992-1996. [31] KAPOOR M, MARTEL-PELLETIER J, LAJEUNESSE D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011; 7(1):33-42. [32] ZHENG W, ZHOU T, ZHANG Y, et al. Simplified α2-macroglobulin as a TNF-α inhibitor for inflammation alleviation in osteoarthritis and myocardial infarction therapy. Biomaterials. 2023;301:122247. [33] YU Y, KIM SM, PARK K, et al. Therapeutic Nanodiamonds Containing Icariin Ameliorate the Progression of Osteoarthritis in Rats. Int J Mol Sci. 2023;24(21):15977. [34] RAPP AE, ZAUCKE F. Cartilage extracellular matrix-derived matrikines in osteoarthritis. Am J Physiol Cell Physiol. 2023;324(2):C377-C394. [35] ROUGHLEY PJ, MORT JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop. 2014;1(1):8. [36] HU Q, ECKER M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int J Mol Sci. 2021;22(4):1742. [37] JIANG X, STOCKWELL BR, CONRAD M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-282. [38] LIU MR, ZHU WT, PEI DS. System Xc-: a key regulatory target of ferroptosis in cancer. Invest New Drugs. 2021;39(4):1123-1131. [39] WU X, LI Y, ZHANG S, et al. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11(7):3052-3059. [40] LIU J, KANG R, TANG D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289(22):7038-7050. [41] LIANG D, MINIKES AM, JIANG X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82(12):2215-2227. [42] WANG S, LI W, ZHANG P, et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J Adv Res. 2022;41:63-75. [43] MIAO Y, CHEN Y, XUE F, et al. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine. 2022;76:103847. [44] ZHANG X, HOU L, GUO Z, et al. Lipid peroxidation in osteoarthritis: focusing on 4-hydroxynonenal, malondialdehyde, and ferroptosis. Cell Death Discov. 2023;9(1):320. [45] XU R, WANG W, ZHANG W. Ferroptosis and the bidirectional regulatory factor p53. Cell Death Discov. 2023;9(1):197. [46] KOPPULA P, ZHUANG L, GAN B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12(8):599-620. [47] GONG Z, WANG Y, LI L, et al. Cardamonin alleviates chondrocytes inflammation and cartilage degradation of osteoarthritis by inhibiting ferroptosis via p53 pathway. Food Chem Toxicol. 2023;174:113644. [48] FAN Z, WIRTH AK, CHEN D, et al. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6(8):e371. [49] INGOLD I, BERNDT C, SCHMITT S, et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172(3):409-422.e21. [50] LIM JKM, DELAIDELLI A, MINAKER SW, et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci U S A. 2019;116(19):9433-9442. [51] XIAO J, LUO C, LI A, et al. Icariin inhibits chondrocyte ferroptosis and alleviates osteoarthritis by enhancing the SLC7A11/GPX4 signaling. Int Immunopharmacol. 2024;133:112010. [52] ZENG C, LIN J, ZHANG K, et al. SHARPIN promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/SLC7A11/GPX4 signaling. Cancer Sci. 2022;113(11):3766-3775. |
| [1] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [2] | Zhao Nannan, Li Yanjie, Qin Hewei, Zhu Bochao, Ding Huimin, Xu Zhenhua. Changes in ferroptosis in hippocampal neurons of vascular dementia model rats treated with Tongmai Kaiqiao Pill [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1401-1407. |
| [3] | Zhang Mingyang, Yang Xinling. Verbascoside inhibits Erastin-induced ferroptosis of dopaminergic nerve cell line MN9D cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1408-1413. |
| [4] | Wang Mi, Ma Shujie, Liu Yang, Qi Rui. Identification and validation of characterized gene NFE2L2 for ferroptosis in ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1466-1474. |
| [5] | Gao Yang, Qin Hewei, Liu Dandan. ACSL4 mediates ferroptosis and its potential role in atherosclerotic cardiovascular disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1239-1247. |
| [6] | Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898. |
| [7] | Xu Tianjie, Fan Jiaxin, Guo Xiaoling, Jia Xiang, Zhao Xingwang, Liu kainan, Wang Qian. Metformin exerts a protective effect on articular cartilage in osteoarthritis rats by inhibiting the PI3K/AKT/mTOR pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1003-1012. |
| [8] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| [9] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [10] | Li Tingyue, Guo Qian, He Wenxi, Wu Jiayuan. Long noncoding RNA TP53TG1 promotes odontogenic and osteogenic differentiation of stem cells from the apical papilla [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7776-7782. |
| [11] | Ma Weibang, Xu Zhe, Yu Qiao, Ouyang Dong, Zhang Ruguo, Luo Wei, Xie Yangjiang, Liu Chen. Screening and cytological validation of cartilage degeneration-related genes in exosomes from osteoarthritis synovial fluid [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7783-7789. |
| [12] | Wang Chen, Zhang Weinan, Shen Jining, Liu Fan, Yuan Jishan, Liu Yake. Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7310-7317. |
| [13] | Pan Yu, Zhao Renfeng, Li Xingping, Zhang Chengdong, Shi Feng, Pu Chao, Luo Xuwei, Xiao Dongqin. Iron overload induces ferroptosis in osteoblast precursor cells and inhibits osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6381-6390. |
| [14] | Yu Qinghe, Cai Ziming, Wu Jintao, Ma Pengfei, Zhang Xin, Zhou Longqian, Wang Yakun, Lin Xiaoqin, Lin Wenping. Vanillic acid inhibits inflammatory response and extracellular matrix degradation of endplate chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6391-9397. |
| [15] | Zhou Ying, Tian Yong, Zhong Zhimei, Gu Yongxiang, Fang Hao. Inhibition of tumor necrosis factor receptor associated factor 6 regulates mTORC1/ULK1 signaling and promotes autophagy to improve myocardial injury in sepsis mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6434-6440. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||