Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (36): 7776-7782.doi: 10.12307/2025.563
Previous Articles Next Articles
Long noncoding RNA TP53TG1 promotes odontogenic and osteogenic differentiation of stem cells from the apical papilla
Li Tingyue1, 2, 3, Guo Qian2, He Wenxi3, Wu Jiayuan1
- 1Stomatological Medical School of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2State Key Laboratory of Military Stomatology, Key Laboratory of Shaanxi Province Stomatology, Department of Dental Pulp Diseases, The Third Affiliated Hospital of Air Force Medical University, Xi’an 710032, Shaanxi Province, China; 3Department of Stomatology, Special Medical Center, Air Force Medical University, Beijing 100142, China
-
Received:2024-09-11Accepted:2024-11-13Online:2025-12-28Published:2025-03-08 -
Contact:Wu Jiayuan, MD, Chief physician, Master’s supervisor, Stomatological Medical School of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Co-corresponding author: He Wenxi, MD, Chief physician, Master’s supervisor, Department of Stomatology, Special Medical Center, Air Force Medical University, Beijing 100142, China -
About author:Li Tingyue, Master candidate, Stomatological Medical School of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; State Key Laboratory of Military Stomatology, Key Laboratory of Shaanxi Province Stomatology, Department of Dental Pulp Diseases, The Third Affiliated Hospital of Air Force Medical University, Xi’an 710032, Shaanxi Province, China; Department of Stomatology, Special Medical Center, Air Force Medical University, Beijing 100142, China -
Supported by:National Natural Science Foundation of China, No. 82460190 (to WJY); Guizhou Science and Technology Plan Project, No. ZK[2022]638 (to WJY); Beijing Natural Science Foundation, No. 7242140 (to HWX); Shaanxi Natural Science Basic Research Plan - Key Project, No. 2022JZ-42 (to HWX)
CLC Number:
Cite this article
Li Tingyue, Guo Qian, He Wenxi, Wu Jiayuan. Long noncoding RNA TP53TG1 promotes odontogenic and osteogenic differentiation of stem cells from the apical papilla[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7776-7782.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
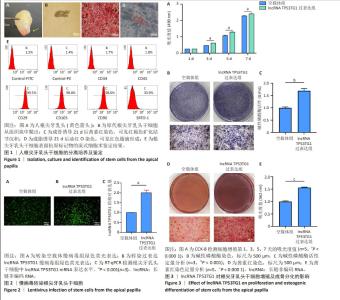
2.1 根尖牙乳头干细胞的分离培养及鉴定结果 收集根尖孔未闭合完全的年轻恒牙,根尖部可见质地坚韧的根尖牙乳头组织(图1A)。组织块消化培养5 d后,镜下可见梭形细胞从组织块中央呈放射状爬出,即为原代根尖牙乳头干细胞(图1B)。根尖牙乳头干细胞经成骨诱导21 d后,茜素红染色镜下可观察到红褐色矿化结节沉积(图1C);经成脂诱导21 d后,油红O染色镜下可见红色脂滴形成(图1D),表明获得的根尖牙乳头干细胞具有多向分化潜能。流式细胞术结果显示,根尖牙乳头干细胞高表达间充质干细胞标志物CD29、CD105、CD90和STRO-1,而不表达造血干细胞标志物CD34、CD45(图1E),符合根尖牙乳头干细胞免疫表型特点。 2.2 慢病毒转染诱导根尖牙乳头干细胞中lncRNA TP53TG1的表达 利用慢病毒转染技术,将携带绿色荧光标签的空载体慢病毒或过表达lncRNA TP53TG1的慢病毒转染至根尖牙乳头干细胞,24 h后通过嘌呤霉素筛选慢病毒感染成功的根尖牙乳头干细胞,72 h后通过荧光显微镜观察到两组均强烈表达绿色荧光(图2A,B)。此外,相比于空载体慢病毒组,lncRNA TP53TG1过表达组根尖牙乳头干细胞中lncRNA TP53TG1 mRNA表达显著增高(图2C,P < 0.001),可用于后续实验。 2.3 过表达lncRNA TP53TG1促进根尖牙乳头干细胞增殖和成骨分化 使用CCK-8法检测过表达lncRNA TP53TG1对根尖牙乳头干细胞增殖的影响。结果显示,从第3天开始,过表达lncRNA TP53TG1显著增强根尖牙乳头干细胞的增殖能力(图3A,P < 0.000 1)。碱性磷酸酶染色结果显示,与空载体组相比,过表达lncRNA TP53TG1组形成颜色更深的蓝紫色沉淀(图3B),碱性磷酸酶活性检测结果也显示过表达lncRNA TP53TG1显著增强根尖牙乳头干细胞中碱性磷酸酶活性(图3C,P < 0.001)。茜素红染色结果显示lncRNA TP53TG1过表达组根尖牙乳头干细胞形成更多的红褐色矿化结节(图3D,E,P < 0.000 1)。 "

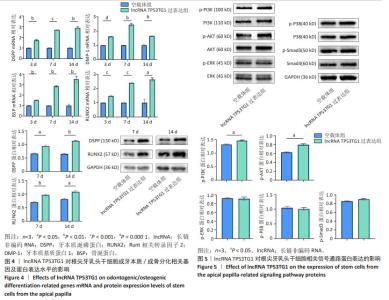
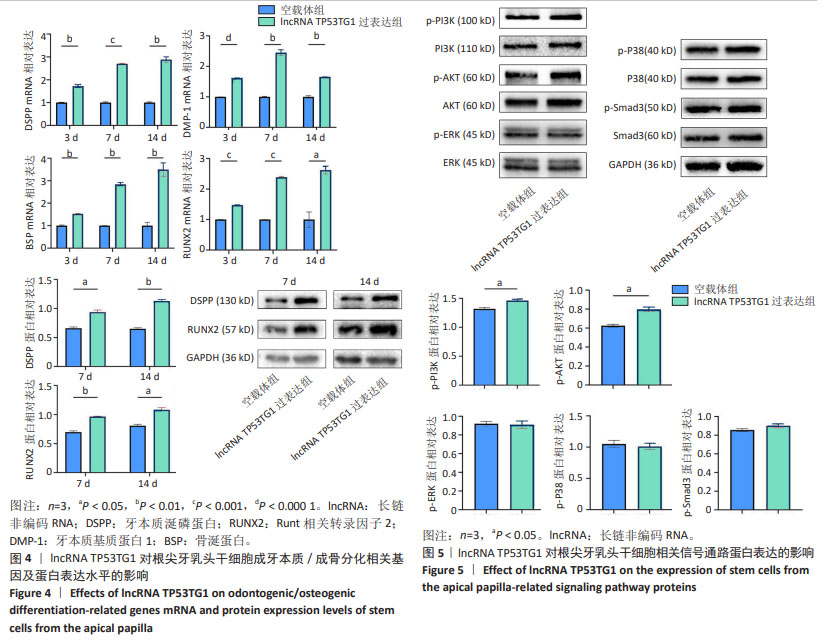
2.4 过表达lncRNA TP53TG1诱导根尖牙乳头干细胞成牙本质/成骨向分化相关指标的表达 RT-qPCR结果显示,矿化诱导3,7,14 d,与空载体组相比,lncRNA TP53TG1过表达组根尖牙乳头干细胞成牙本质向分化相关基因DSPP和DMP-1以及成骨向分化相关基因BSP和RUNX2的mRNA表达水平上调(P < 0.05)。同时,Western blot 结果显示,矿化诱导7,14 d,与空载体组相比,lncRNA TP53TG1过表达组DSPP和RUNX2的蛋白表达水平也上调,差异有显著性意义(P < 0.05),见图4。 2.5 过表达lncRNA TP53TG1激活PI3K/AKT信号通路 Western blot结果显示,与空载体组相比,过表达lncRNA TP53TG1后多条信号通路总蛋白表达水平基本相同,但PI3K及AKT的磷酸化蛋白水平升高(P < 0.05),而ERK1/2、p38、Smad3磷酸化蛋白的表达并未表现出有统计学差异(P > 0.05),见图5,提示lncRNA TP53TG1可通过上调根尖牙乳头干细胞中PI3K及AKT的磷酸化水平而激活PI3K/AKT信号通路。 "
| [1] HEBOYAN A, AVETISYAN A, KAROBARI MI, et al. Tooth root resorption: A review. Sci Prog. 2022;105(3):368504221109217. [2] MURRAY PE. Review of guidance for the selection of regenerative endodontics, apexogenesis, apexification, pulpotomy, and other endodontic treatments for immature permanent teeth. Int Endod J. 2023;56 Suppl 2:188-199. [3] SHAH A, PEACOCK R, ELIYAS S. Pulp therapy and root canal treatment techniques in immature permanent teeth: an update. Br Dent J. 2022;232(8):524-530. [4] LIU Q, GAO Y, HE J. Stem Cells from the Apical Papilla (SCAPs): Past, Present, Prospects, and Challenges. Biomedicines. 2023;11(7):2047. [5] DRIESEN RB, GERVOIS P, VANGANSEWINKEL T, et al. Unraveling the Role of the Apical Papilla During Dental Root Maturation. Front Cell Dev Biol. 2021;9:665600. [6] GAO X, RUZBARSKY JJ, LAYNE JE, et al. Stem Cells and Bone Tissue Engineering. Life (Basel). 2024;14(3):287. [7] KHORKOVA O, STAHL J, JOJI A, et al. Long non-coding RNA-targeting therapeutics: discovery and development update. Expert Opin Drug Discov. 2023;18(9): 1011-1029. [8] BRIDGES MC, DAULAGALA AC, KOURTIDIS A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045. [9] CHEN Z, ZHANG K, QIU W, et al. Genome-wide identification of long noncoding RNAs and their competing endogenous RNA networks involved in the odontogenic differentiation of human dental pulp stem cells. Stem Cell Res Ther. 2020;11(1):114. [10] GU Y, BAI Y. LncRNA MALAT1 promotes osteogenic differentiation through the miR-93-5p/SMAD5 axis. Oral Dis. 2024;30(4):2398-2409. [11] ZHANG Z, HE Q, YANG S, et al. Mechanical force-sensitive lncRNA SNHG8 inhibits osteogenic differentiation by regulating EZH2 in hPDLSCs. Cell Signal. 2022;93: 110285. [12] TAKEI Y, ISHIKAWA S, TOKINO T, et al. Isolation of a novel TP53 target gene from a colon cancer cell line carrying a highly regulated wild-type TP53 expression system. Genes Chromosomes Cancer. 1998;23(1):1-9. [13] LU Q, GUO Q, XIN M, et al. LncRNA TP53TG1 Promotes the Growth and Migration of Hepatocellular Carcinoma Cells via Activation of ERK Signaling. Noncoding RNA. 2021; 7(3):52. [14] LU Q, XIN M, GUO Q, et al. Knockdown of lncRNA TP53TG1 Enhances the Efficacy of Sorafenib in Human Hepatocellular Carcinoma Cells. Noncoding RNA. 2022; 8(4):61. [15] ZHANG Y, YANG H, DU Y, et al. Long noncoding RNA TP53TG1 promotes pancreatic ductal adenocarcinoma development by acting as a molecular sponge of microRNA-96. Cancer Sci. 2019;110(9):2760-2772. [16] LIAO D, LIU X, YUAN X, et al. Long non-coding RNA tumor protein 53 target gene 1 promotes cervical cancer development via regulating microRNA-33a-5p to target forkhead box K2. Cell Cycle. 2022;21(6):572-584. [17] FERRER J, DIMITROVA N. Transcription regulation by long non-coding RNAs: mechanisms and disease relevance. Nat Rev Mol Cell Biol. 2024;25(5):396-415. [18] 代子寒,王明浩,王胜朝,等. lncRNA TP53TG1在牙髓中的表达和对牙髓干细胞转录炎症因子的影响[J].空军军医大学学报,2024,45(7):833-837. [19] DU L, YANG P, GE S. Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method. J Dent Sci. 2016;11(3): 304-314. [20] LIU F, WANG X, XU J, et al. Preliminary study on the mechanism by which exosomes derived from human exfoliated deciduous teeth improve the proliferation and osteogenic inhibitory effect of glucocorticoid-induced BMSCs. Gene. 2024;923:148575. [21] 甄蕾,刘宏伟.人牙周膜干细胞的初步鉴定及体外成骨诱导[J].口腔颌面外科杂志,2008,18(5):318-322. [22] KUMAR A, RAIK S, SHARMA P, et al. Primary Culture of Dental Pulp Stem Cells. J Vis Exp. 2023;(195):e65223. [23] SHIELDS EJ, PETRACOVICI AF, BONASIO R. lncRedibly versatile: biochemical and biological functions of long noncoding RNAs. Biochem J. 2019;476(7):1083-1104. [24] GEISLER S, COLLER J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14(11):699-712. [25] YANG L, LIN C, LIU W, et al. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell. 2011;147(4):773-788. [26] YU S, GUO J, YANG D, et al. The ATF4-regulated LncRNA MALAT1 promotes odontoblastic differentiation of human dental pulp stem cells via histone demethylase JMJD3: An in vitro study. Int Endod J. 2024;57(1):50-63. [27] YANG C, XU X, LIN P, et al. Overexpression of long noncoding RNA MCM3AP-AS1 promotes osteogenic differentiation of dental pulp stem cells via miR-143-3p/IGFBP5 axis. Hum Cell. 2022;35(1):150-162. [28] WANG D, ZHU N, XIE F, et al. Long non-coding RNA IGFBP7-AS1 promotes odontogenic differentiation of stem cells from human exfoliated deciduous teeth through autophagy: An in vitro study. Arch Oral Biol. 2022;141:105492. [29] TU S, CHEN Y, FENG Y, et al. lncRNA CYTOR Facilitates Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Modulating SOX11 via Sponging miR-6512-3p. Stem Cells Int. 2023;2023:5671809. [30] SUFIANOV A, BEILERLI A, BEGLIARZADE S, et al. The role of noncoding RNAs in the osteogenic differentiation of human periodontal ligament-derived cells. Noncoding RNA Res. 2022;8(1):89-95. [31] ARORA S, COOPER PR, RATNAYAKE JT, et al. A critical review of in vitro research methodologies used to study mineralization in human dental pulp cell cultures. Int Endod J. 2022;55 Suppl 1(Suppl 1):3-13. [32] LIM D, WU KC, LEE A, et al. DSPP dosage affects tooth development and dentin mineralization. PLoS One. 2021;16(5):e0250429. [33] SUZUKI S, HARUYAMA N, NISHIMURA F, et al. Dentin sialophosphoprotein and dentin matrix protein-1: Two highly phosphorylated proteins in mineralized tissues. Arch Oral Biol. 2012;57(9):1165-1175. [34] CAMILLERI S, MCDONALD F. Runx2 and dental development. Eur J Oral Sci. 2006; 114(5):361-373. [35] VIJAYKUMAR A, DYRKACZ P, VIDOVIC-ZDRILIC I, et al. Expression of BSP-GFPtpz Transgene during Osteogenesis and Reparative Dentinogenesis. J Dent Res. 2020; 99(1):89-97. [36] ZHANG W, YELICK PC. Tooth Repair and Regeneration: Potential of Dental Stem Cells. Trends Mol Med. 2021;27(5):501-511. [37] WEN X, JIAO L, TAN H. MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration. Int J Mol Sci. 2022;23(3):1464. [38] LAV R, KRIVANEK J, ANTHWAL N, et al. Wnt signaling from Gli1-expressing apical stem/progenitor cells is essential for the coordination of tooth root development. Stem Cell Reports. 2023;18(4):1015-1029. [39] TAKIMOTO K, WIDBILLER M, DIOGENES A. Expression of Toll-like Receptors in Stem Cells of the Apical Papilla and Its Implication for Regenerative Endodontics. Cells. 2023;12(20):2502. [40] LI Z, GE X, LU J, et al. MiR-141-3p regulates proliferation and senescence of stem cells from apical papilla by targeting YAP. Exp Cell Res. 2019;383(2):111562. [41] JIN L, CAO Y, YU G, et al. SFRP2 enhances the osteogenic differentiation of apical papilla stem cells by antagonizing the canonical WNT pathway. Cell Mol Biol Lett. 2017;22:14. [42] ZHAO Q, REN H, WANG N, et al. NOTUM plays a bidirectionally modulatory role in the odontoblastic differentiation of human stem cells from the apical papilla through the WNT/β-catenin signaling pathway. Arch Oral Biol. 2024;160:105896. [43] WANG Y, LU Y, LI Z, et al. Oestrogen receptor α regulates the odonto/osteogenic differentiation of stem cells from apical papilla via ERK and JNK MAPK pathways. Cell Prolif. 2018;51(6):e12485. [44] PANG X, ZHUANG Y, LI Z, et al. Intermittent Administration of Parathyroid Hormone Enhances Odonto/Osteogenic Differentiation of Stem Cells from the Apical Papilla via JNK and P38 MAPK Pathways. Stem Cells Int. 2020;2020:5128128. [45] LIU Z, LIN Y, FANG X, et al. Epigallocatechin-3-Gallate Promotes Osteo-/Odontogenic Differentiation of Stem Cells from the Apical Papilla through Activating the BMP-Smad Signaling Pathway. Molecules. 2021;26(6):1580. [46] CHENG Q, ZENG K, KANG Q, et al. The Antimicrobial Peptide LL-37 Promotes Migration and Odonto/Osteogenic Differentiation of Stem Cells from the Apical Papilla through the Akt/Wnt/β-catenin Signaling Pathway. J Endod. 2020;46(7): 964-972. [47] TANAKA Y, SONODA S, YAMAZA H, et al. Acetylsalicylic Acid Treatment and Suppressive Regulation of AKT Accelerate Odontogenic Differentiation of Stem Cells from the Apical Papilla. J Endod. 2019;45(5):591-598.e6. [48] WANG Z, CHEN C, SUN L, et al. Fibroblast growth factor 2 promotes osteo/odontogenic differentiation in stem cells from the apical papilla by inhibiting PI3K/AKT pathway. Sci Rep. 2024;14(1):19354. [49] TANAKA Y, SONODA S, YAMAZA H, et al. Suppression of AKT-mTOR signal pathway enhances osteogenic/dentinogenic capacity of stem cells from apical papilla. Stem Cell Res Ther. 2018;9(1):334. [50] CHEN X, LIU JY, YUE L, et al. Phosphatidylinositol 3-Kinase and Protein Kinase C Signaling Pathways Are Involved in Stromal Cell-derived Factor-1α-mediated Transmigration of Stem Cells from Apical Papilla. J Endod. 2016;42(7): 1076-1081. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [3] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [4] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [5] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [6] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [7] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [8] | Wang Xuanqiang, Zhang Wenyang, Li Yang, Kong Weiqian, Li Wei, Wang Le, Li Zhongshan, Bai Shi. Effects of chronic exposure to low-frequency pulsed magnetic fields on contractility and morphology of the quadriceps muscle in healthy adults [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1634-1642. |
| [9] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [10] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [11] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [12] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [13] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [14] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [15] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||