Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (36): 7709-7718.doi: 10.12307/2025.535
Previous Articles Next Articles
Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells
Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin
- Department of Orthopedics, First Affiliated Hospital of Soochow University, Institute of Orthopedics of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2024-04-15Accepted:2024-06-21Online:2025-12-28Published:2025-03-01 -
Contact:Shi Qin, MD, Professor, Department of Orthopedics, First Affiliated Hospital of Soochow University, Institute of Orthopedics of Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Zhang Xiongjinfu, Department of Orthopedics, First Affiliated Hospital of Soochow University, Institute of Orthopedics of Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 82172485 (to SQ)
CLC Number:
Cite this article
Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
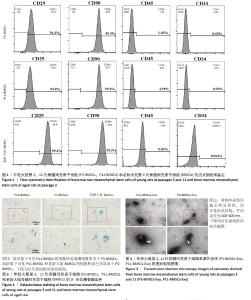
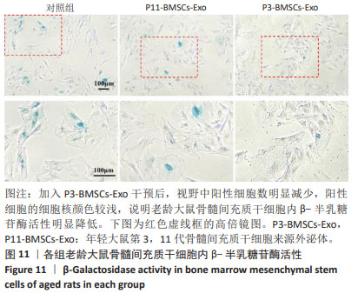
2.1 骨髓间充质干细胞的鉴定结果 流式细胞术结果显示,年轻大鼠第3,11代骨髓间充质干细胞、老龄大鼠骨髓间充质干细胞阳性指标CD90的阳性百分比分别为(99.50±0.01)%,(99.400±0.003)%,(99.900±0.006)%,CD29的阳性百分比分别为(94.40±0.06)%,(94.00±0.05)%,(99.40±0.01)%;阴性指标CD34的阳性百分比分别为(0.030±0.007)%,(0.93±0.08)%,(0.004±0.002)%,CD45的阳性百分比分别为(1.43±0.20)%,(4.55±0.01)%,(0.46±0.01)%,均小于5%。该鉴定结果说明提取培养的骨髓间充质干细胞纯度达到 90%以上,见图1。 2.2 年轻大鼠第3,11代骨髓间充质干细胞和老龄大鼠第3代骨髓间充质干细胞的β-半乳糖苷酶染色结果 低倍镜下可见第11代骨髓间充质干细胞的细胞核蓝染细胞数量多于第3代骨髓间充质干细胞,高倍镜下可见第11代骨髓间充质干细胞的细胞核着色明显深于第3代骨髓间充质干细胞,见图2。 2.3 第3,11代骨髓间充质干细胞来源外泌体形貌 透射电镜观察显示,第3,11代骨髓间充质干细胞来源外泌体具有非常明显的膜边界、直径150 nm左右的杯状结构,也有部分在200 nm以下、较难鉴别的其他形状外泌体。两种外泌体的形貌无明显差别,可排除大小、形态对后续实验的干扰,见图3。 "

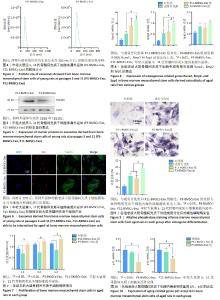
2.4 第3,11代骨髓间充质干细胞来源外泌体的粒径分布 纳米颗粒分析结果显示,第3,11代骨髓间充质干细胞来源外泌体的粒径大小主要分布在150 nm 左右,检测结果呈现单峰,说明提取出的外泌体纯度较高,见图4。 2.5 第3,11代骨髓间充质干细胞来源外泌体的标志蛋白表达 第3,11代骨髓间充质干细胞来源外泌体均表达CD63和TSG101,符合外泌体的表征验证,见图5。 2.6 老龄大鼠骨髓间充质干细胞对年轻大鼠第3,11代骨髓间充质干细胞来源外泌体的内化 倒置荧光显微镜观察结果显示,年轻大鼠第3,11代骨髓间充质干细胞来源外泌体均能被老龄大鼠骨髓间充质干细胞摄取,二者内化情况一致,无明显差别,外泌体均聚集在老龄大鼠骨髓间充质干细胞的细胞质和细胞核周围,进一步表明老龄大鼠骨髓间充质干细胞能够内化年轻大鼠第3,11代骨髓间充质干细胞来源外泌体,被摄取的外泌体能够进一步进入细胞核内发挥生物学作用,见图6。 2.7 第3代骨髓间充质干细胞来源外泌体促进老龄大鼠骨髓间充质干细胞的增殖 老龄大鼠骨髓间充质干细胞接种培养1,3,5 d后,能够正常增殖,并且加入第3代骨髓间充质干细胞来源外泌体处理后老龄大鼠骨髓间充质干细胞的增殖速度增加,加入第11代骨髓间充质干细胞来源外泌体后老龄大鼠骨髓间充质干细胞的增殖速度下降。上述结果表明第3代骨髓间充质干细胞来源外泌体能够促进老龄大鼠骨髓间充质干细胞的增殖,见图7。 2.8 第3代骨髓间充质干细胞来源外泌体促进老龄大鼠骨髓间充质干细胞成骨分化相关基因的表达 与第11代骨髓间充质干细胞来源外泌体组相比,第3代骨髓间充质干细胞来源外泌体组Bmp2、Spp1和Runx2的mRNA表达均上调,见图8。 2.9 第3代骨髓间充质干细胞来源外泌体提高老龄大鼠骨髓间充质干细胞成骨分化后的碱性磷酸酶活性 碱性磷酸酶染色结果表明,加入第3代骨髓间充质干细胞来源外泌体后,老龄大鼠骨髓间充质干细胞的碱性磷酸酶表达量显著增加,见图9。 2.10 第3代骨髓间充质干细胞来源外泌体降低老龄大鼠骨髓间充质干细胞衰老相关基因表达 与第11代骨髓间充质干细胞来源外泌体组相比,第3代骨髓间充质干细胞来源外泌体组p21的mRNA表达下调了约48%,p16的mRNA表达下调了约30%(P < 0.05),见图10。 "


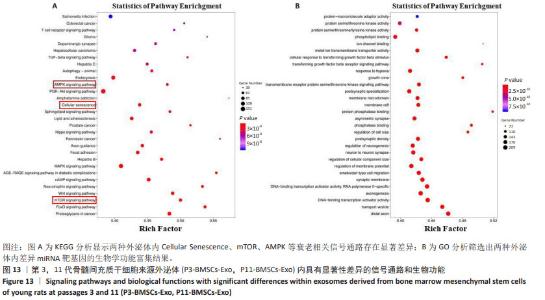
2.12 第3代骨髓间充质干细胞来源外泌体逆转老龄大鼠骨髓间充质干细胞衰老相关信号 利用Small RNA测序比较两种外泌体处理后老龄大鼠骨髓间充质干细胞内miRNA表达的差异,在 Fold Change≥2、P < 0.05的条件下,筛选具有显著性差异的miRNA,通过Targetscan 8.0寻找具有显著性表达差异的miRNA的靶基因,筛选靶基因中与细胞衰老调控相关的基因,结合KEGG分析结果,将靶基因所在细胞通路与KEGG分析结果对应,结果显示mTOR、AMPK等衰老相关信号通路均有显著性差异miRNA富集,这表明第3代骨髓间充质干细胞来源外泌体内部存在衰老调控相关的miRNA,见图12A。火山图显示,与第3代骨髓间充质干细胞来源外泌体相比,第11代骨髓间充质干细胞来源外泌体中表达上调的miRNA有20个,表达下调的miRNA有 22个,见图12B。热图显示第3代骨髓间充质干细胞来源外泌体中显著上调的miRNA是 let-7c-5p、let-7b-5p、miR-320-3p和 miR-26a-5p,进一步寻找上述miRNA的靶基因,分析其所在信号通路。通过GO分析结果表明,两种外泌体内的差异性miRNA与细胞膜运动、生长因子运输、DNA结合及转录等重要细胞学作用相关,见图13。"

| [1] CAO Y, RUAN J, KANG J, et al. Extracellular Vesicles in Infrapatellar Fat Pad from Osteoarthritis Patients Impair Cartilage Metabolism and Induce Senescence. Adv Sci (Weinh). 2024;11(3):e2303614. [2] PARTRIDGE L, DEELEN J, SLAGBOOM PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45-56. [3] BEHR LC, SIMM A, KLUTTIG A, et al. 60 years of healthy aging: On definitions, biomarkers, scores and challenges. Ageing Res Rev. 2023;88:101934. [4] REID IR. A broader strategy for osteoporosis interventions. Nat Rev Endocrinol. 2020;16(6):333-339. [5] BONE HG, WAGMAN RB, BRANDI ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7): 513-523. [6] BLACK DM, ROSEN CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. 2016;374(3):254-262. [7] AREECKAL AS, KOCHER M, S SD. Current and Emerging Diagnostic Imaging-Based Techniques for Assessment of Osteoporosis and Fracture Risk. IEEE Rev Biomed Eng. 2019;12:254-268. [8] QIN L, LIU W, CAO H, et al. Molecular mechanosensors in osteocytes. Bone Res. 2020;8:23. [9] BUNPETCH V, ZHANG ZY, ZHANG X, et al. Strategies for MSC expansion and MSC-based microtissue for bone regeneration. Biomaterials. 2019;196:67-79. [10] KIERNAN J, DAVIES JE, STANFORD WL. Concise Review: Musculoskeletal Stem Cells to Treat Age-Related Osteoporosis. Stem Cells Transl Med. 2017;6(10):1930-1939. [11] WANG Z, GOH J, DAS DE S, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12(7): 1753-1761. [12] HAN W, YU Y, LIU XY. Local signals in stem cell-based bone marrow regeneration. Cell Res. 2006;16(2):189-195. [13] LI F, ZHOU C, XU L, et al. Effect of Stem Cell Therapy on Bone Mineral Density: A Meta-Analysis of Preclinical Studies in Animal Models of Osteoporosis. PLoS One. 2016;11(2):e0149400. [14] KALLURI R, LEBLEU VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [15] MATHIEU M, MARTIN-JAULAR L, LAVIEU G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17. [16] REN R, TAN XH, ZHAO JH, et al. Bone marrow mesenchymal stem cell-derived exosome uptake and retrograde transport can occur at peripheral nerve endings. Artif Cells Nanomed Biotechnol. 2019; 47(1):2918-2929. [17] YING C, WANG R, WANG Z, et al. BMSC-Exosomes Carry Mutant HIF-1α for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Front Bioeng Biotechnol. 2020;8:565561. [18] BEI HP, HUNG PM, YEUNG HL, et al. Bone-a-Petite: Engineering Exosomes towards Bone, Osteochondral, and Cartilage Repair. Small. 2021;17(50):e2101741. [19] YANG D, ZHANG W, ZHANG H, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684-3707. [20] YU W, HURLEY J, ROBERTS D, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466-477. [21] FAFIÁN-LABORA JA, RODRÍGUEZ-NAVARRO JA, O’LOGHLEN A. Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metab. 2020;32(1):71-86.e5. [22] ZHANG S, CHUAH SJ, LAI RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [23] ZHOU B, XU K, ZHENG X, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5(1):144. [24] HU N, CAI Z, JIANG X, et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175-186. [25] RUAN Y, KATO H, TAGUCHI Y, et al. Irradiation by high-intensity red light-emitting diode enhances human bone marrow mesenchymal stem cells osteogenic differentiation and mineralization through Wnt/β-catenin signaling pathway. Lasers Med Sci. 2021;36(1):55-65. [26] BHUJEL B, SHIN HE, CHOI DJ, et al. Mesenchymal Stem Cell-Derived Exosomes and Intervertebral Disc Regeneration: Review. Int J Mol Sci. 2022;23(13):7306. [27] AGUAYO-MAZZUCATO C, ANDLE J, LEE TB JR, et al. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019;30(1):129-142.e4. [28] JIN S, WANG Y, WU X, et al. Young Exosome Bio-Nanoparticles Restore Aging-Impaired Tendon Stem/Progenitor Cell Function and Reparative Capacity. Adv Mater. 2023;35(18):e2211602. [29] XIAO Y, XIE X, CHEN Z, et al. Advances in the roles of ATF4 in osteoporosis. Biomed Pharmacother. 2023;169:115864. [30] MORA-RAIMUNDO P, LOZANO D, MANZANO M, et al. Nanoparticles to Knockdown Osteoporosis-Related Gene and Promote Osteogenic Marker Expression for Osteoporosis Treatment. ACS Nano. 2019; 13(5):5451-5464. [31] HU Y, TIAN H, CHEN W, et al. The Critical Role of The Piezo1/β-catenin/ATF4 Axis on The Stemness of Gli1+ BMSCs During Simulated Microgravity-Induced Bone Loss. Adv Sci (Weinh). 2023; 10(32):e2303375. [32] ANKRUM JA, ONG JF, KARP JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252-260. [33] ROMBOUTS WJ, PLOEMACHER RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160-170. [34] YÁÑEZ-MÓ M, SILJANDER PR, ANDREU Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [35] THÉRY C, WITWER KW, AIKAWA E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [36] WITWER KW, THÉRY C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. 2019; 8(1):1648167. [37] ZHA Y, LI Y, LIN T, et al. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11(1):397-409. [38] HAN C, SUN X, LIU L, et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016;2016:7653489. [39] WU J, KUANG L, CHEN C, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87-100. [40] JIANG YL, WANG ZX, LIU XX, et al. The Protective Effects of Osteocyte-Derived Extracellular Vesicles Against Alzheimer’s Disease Diminished with Aging. Adv Sci (Weinh). 2022;9(17):e2105316. [41] PLAKHOVA N, PANAGOPOULOS V, VANDYKE K, et al. Mesenchymal stromal cell senescence in haematological malignancies. Cancer Metastasis Rev. 2023; 42(1):277-296. [42] BRUDER SP, JAISWAL N, HAYNESWORTH SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278-294. [43] YANG YK, OGANDO CR, WANG SEE C, et al. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9(1):131. [44] THIND A, WILSON C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. [45] QIU M, ZHAI S, FU Q, et al. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MicroRNA-150-3p Promotes Osteoblast Proliferation and Differentiation in Osteoporosis. Hum Gene Ther. 2021;32(13-14):717-729. [46] YANG X, YANG J, LEI P, et al. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany NY). 2019;11(20):8777-8791. [47] GONZÁLEZ A, HALL MN, LIN SC, et al. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020;31(3):472-492. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [3] | Miao Jiahang, Ma Sheng, Li Qupeng, Yu Huilin, Hu Tianyu, Gao Xiao, Feng Hu. Cervical lordosis ratio can be used as a decision-making indicator for selection of posterior surgical approach for multi-level cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1796-1802. |
| [4] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [5] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [6] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [7] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [8] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [9] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [10] | Weng Zongqin, Zhao Hailong. Mechanism of exosomal miRNA involved in tumor chemotherapy resistance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1504-1511. |
| [11] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [12] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [13] | Li Yueyao, Zhang Min, Yang Jiaju. Cistanoside A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1144-1151. |
| [14] | Zheng Lin, Jin Wenjun, Luo Shanshan, Huang Rui, Wang Jie, Cheng Yuting, An Zheqing, Xiong Yue, Gong Zipeng, Liao Jian. Eucommia ulmoides promotes alveolar bone formation in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1159-1167. |
| [15] |
Huang Xiaobin, Ge Jirong, Li Shengqiang, Xie Lihua, Huang Jingwen, He Yanyan, Xue Lipeng.
Mechanisms of different yin nourishing and kidney tonifying methods on osteoclastysis pathway in ovariectomized rats #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1214-1219.
|
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||