Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7385-7392.doi: 10.12307/2025.496
Previous Articles Next Articles
Decellularized tendon scaffold: a biomedical material for tendon injury repair
Yi Xiaoding, Zhang Di, Guo Hong, Qing Liang, Zhao Tianyu
- Department of Rehabilitation, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine/TCM Hospital of Sichuan Province, Chengdu 610072, Sichuan Province, China
-
Received:2024-07-25Accepted:2024-09-24Online:2025-12-08Published:2025-01-17 -
Contact:Zhao Tianyu, MS, Technician, Rehabilitation therapist, Department of Rehabilitation, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine/TCM Hospital of Sichuan Province, Chengdu 610072, Sichuan Province, China -
About author:Yi Xiaoding, MS, Attending physician, Rehabilitation physician, Department of Rehabilitation, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine/TCM Hospital of Sichuan Province, Chengdu 610072, Sichuan Province, China -
Supported by:2023 Science and Technology Development Fund of Chengdu University of Traditional Chinese Medicine Affiliated Hospital, No. 23TS20 (to ZTY); 2022 Science and Technology Development Fund of Chengdu University of Traditional Chinese Medicine Affiliated Hospital, No. 22ZL10 (to GH)
CLC Number:
Cite this article
Yi Xiaoding, Zhang Di, Guo Hong, Qing Liang, Zhao Tianyu. Decellularized tendon scaffold: a biomedical material for tendon injury repair[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7385-7392.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
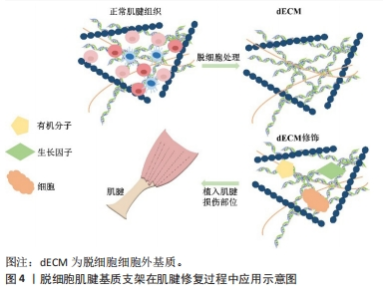
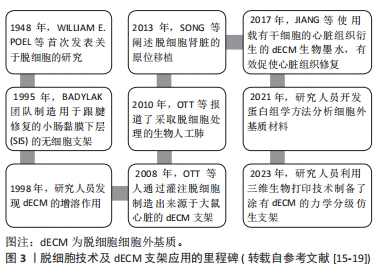
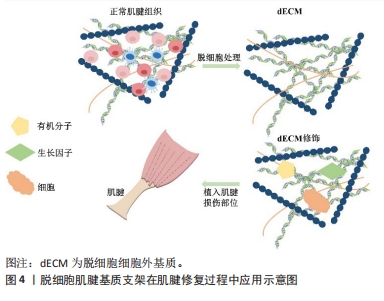
2.3 脱细胞肌腱支架的生物特性及特点 组织工程与再生医学中常使用的生物材料是支架,它能够连接组织和细胞,模仿天然组织内环境,为组织再生打下坚实的基础。正常肌腱细胞外基质能够保持各胶原的比例,维持肌腱细胞结构稳定,还参与肌腱细胞的分化、再生和黏附,是肌腱组织内最为重要的结构之一[21]。脱细胞肌腱基质支架以脱细胞细胞外基质为基底被植入到损伤部位,外源性细胞外基质随着时间的推移逐渐降解,而肌腱细胞分泌新细胞外基质,促进组织修复和愈合[22]。图4为脱细胞肌腱基质支架在肌腱修复中的应用流程示意图。总的来说,脱细胞肌腱基质支架通常具备以下特征:高强度张力、高耐磨性、高孔隙率的三维结构、生物相容性、较好的生物力学性能及再生能力[23-24]。"

2.3.1 生物降解性 脱细胞肌腱基质支架的主要功能之一是维持胶原纤维的整齐排列,促进细胞增殖和再生,提高组织再生能力,但支架并不是永久植入物,这就需要脱细胞肌腱基质支架具备一定的降解性和无毒性,同时它的降解过程不应该干扰其他器官或细胞的活动。支架的降解是必要过程,降解产物能够招募宿主细胞,有助于细胞增殖、组织重塑及细胞外基质合成[25]。支架降解速率应当与细胞生长过程速率持平,如果支架降解的速率远远超过肌腱细胞生长和浸润的速率,支架就难以影响细胞容貌变化和形态改变的过程[26-27]。支架降解和组织再生同步进行能够缓和炎症反应等不良影响。随着组织修复的过程进行,脱细胞肌腱基质支架和外源性细胞外基质降解,新宿主细胞分泌和产生新细胞外基质,细胞自身的细胞外基质逐渐形成,最终为肌腱组织再生提供条件。 2.3.2 生物相容性 脱细胞化的过程可以去除细胞和免疫原性物质(DNA、MHC i -复合物、MHC ii -复合物),保留了细胞骨架蛋白、糖蛋白(纤连蛋白、层粘连蛋白、胶原蛋白)、蛋白聚糖(肝素、硫酸软骨素)和弹性蛋白等细胞外基质成分,洗涤剂能够部分消除化学剂附带的有毒化学物质,从而维持组织细胞外基质微结构的完好性,为支架的生物相容性构成基础[25,28]。良好的生物相容性能为组织修复过程中细胞的再生或分化提供前提,维持细胞的正常运作和功能发挥,细胞迁移到组织受损表面,让组织和支架相融合,通过支架的支撑作用形成新的基质[25,29-30],此过程需要保障生物体的安全性,降低炎症、免疫及排斥反应的出现概率。脱细胞肌腱基质支架的生物相容性在实验过程中通过测试材料内的细胞毒性来评价,这为脱细胞肌腱基质支架中的脱细胞化处理奠定基础[31-32]。 2.3.3 生物力学性能 脱细胞肌腱基质支架在清除细胞外基质内部成分后能够基本维持类似天然肌腱内的正常胶原排列结构,保持与天然肌腱类似的刚度、强度、弹性等生物力学性,它必须足够坚固才能维持相关细胞活动(机械转导和行为调节)[30]。制造具备一定机械性能的支架对于肌腱损伤的治疗是一个极大的挑战,因为存在不可忽略的客观原因,譬如年龄,随着年龄的增长,肌腱损伤的恢复时间越来越长,所以支架的性能需与患者的年龄或其他客观性生理活动相适应[30,33]。支架的组成部分与特性也会反映到肌腱组织机械性能上,生物力学性能的维持与支架的孔隙率大小是相辅相成的,部分支架若是由于空隙较小导致细胞的异常增殖分化或血管的延伸拓展,这将会加大支架植入的失败概率,所以支架的机械性能与高孔隙率及合适的孔径相适应才能保证废物排出、细胞浸润等活动的完成[10,30]。目前,NA等[34]开发了一种PET基贴片(PM)治疗肩袖损伤,这种复合贴片具备良好的力学性能,结构平均孔径为652.51 μm,孔隙率为95.43%,显著提高了肌腱成熟评分,再生化胶原直径增粗,同样的孔径和孔隙率是否仍然适用于其他类型的肌腱损伤仍然需要进一步实验研究。 2.4 脱细胞肌腱基质支架促进肌腱损伤修复的机制和作用 2.4.1 抗炎和抗氧化作用 研究表明肌腱损伤修复的过程伴随炎症反应的开启,炎症递质和相关免疫细胞开始发挥作用,它们的活动可能具备协同性,炎症递质通过改变某些细胞的表型调节肌腱基质内部成分的性质,诱导生成更多炎症递质[35]。肌腱损伤修复的炎症期发生于组织损伤后的48 h之内,大量炎症因子(巨噬细胞、单核细胞前体等)向损伤的部位移动,激活局部损伤部位的防御机制,刺激趋化因子的活动,募集肌腱成纤维细胞为后续组织再生提供条件[36-37]。 在肌腱修复早期部分炎症物质发挥作用,其中M1巨噬细胞表型起主要作用,它可以通过分泌部分炎症因子和促炎酶,调节组织炎症反应的过程;而M2巨噬细胞主要是由M1巨噬细胞转化而来,它的含量随着肌腱修复过程逐渐上升,指导干细胞分化、控制细胞外基质沉积和组织重塑的过程 [38-40]。脱细胞肌腱基质支架通过促进部分抗炎因子例如白细胞介素4和白细胞介素10的表达,同时抑制部分炎症因子例如白细胞介素6和白细胞介素1β的分泌来诱导M1巨噬细胞向M2巨噬细胞的极化,提高巨噬细胞 M2/M1 比率,调节肌腱内部环境,影响肌腱组织的再生和结构重塑[41]。 肌腱再生在很大程度上还会受到氧化剂和炎症微环境的影响。活性氧是大部分需氧动物线粒体呼吸时氧代谢的产物,线粒体通过控制其水平影响活性物质含量的变化,在细胞损伤、坏死和增殖等活动中发挥作用[42]。肌腱修复过程中出现的持续性炎症会导致肌腱粘连和活性氧过度积累,而过量的活性氧会影响组织重塑和再生[43]。实验证实,活性氧能够刺激由核因子红细胞相关因子2(nuclear factor-erythroid 2-Related Factor 2,Nrf2)所控制的抗氧化系统,活性氧含量与Nrf2的表达成反比,脱细胞肌腱基质支架通过缓解组织损伤产生的炎症反应的同时降低活性氧的表达以此增加Nrf2的表达,最终促使肌腱修复。在这个过程中伴随抗炎因子例如精氨酸酶1表达上升,促炎因子例如一氧化氮合酶和CD86表达下降,清除体内外过多的活性氧,从而有效延缓炎症反应[41]。然而,为了防止脱细胞肌腱基质支架植入后的炎症反应,脱细胞必须在体内或持续的血管灌注中进行,以便能够删除细胞碎片[15]。综上所述,使用脱细胞肌腱基质支架可以有效减轻肌腱组织损伤时所出现的炎症反应,缩短炎症反应的持续时间,从而进行组织修复[25]。 2.4.2 促进干细胞的增殖和分化 肌腱修复早期的炎症反应会损害干细胞的成腱分化能力,造成其异常分化,从而引起细胞凋亡、基质变性等后果[41]。脱细胞肌腱基质支架自身的特性能很好地规避炎症反应带来的负面影响,首先细胞外基质的脱细胞化处理能够释放部分生长因子从而增强干细胞的分化能力;其次去细胞化肌腱在清除细胞外基质内成分的同时还可以维持细胞外基质微结构的完整性,这能为细胞发挥作用形成前提和背景[25,44]。 在这种天然的仿生微环境下,肌腱源性脱细胞基质通过调节肌腱特异性转录因子Sclaxis(SCX)和Runt相关转录因子2(Runx2)的表达为肌腱组织中肌腱源性干细胞和骨髓源性干细胞向成腱谱系(肌腱或软骨)分化提供诱导性作用,即使是在成骨诱导的条件下也能诱导干细胞的成腱分化[10,45-48]。两种干细胞向肌腱成纤维细胞分化的同时会释放大量转化生长因子β1(TGF-β1),这种因子通过 Wnt信号蛋白/β-连锁蛋白信号通路(Wnt/β-catenin)、 黏着斑激酶/蛋白激酶B/雷帕霉素靶蛋白信号通路 (FAK-Akt-mTOR)、神经钙黏蛋白(N-cadherin)和非典型转化生长因子β信号诱导肌腱源性干细胞和骨髓源性干细胞迁移,这会促进Ⅰ型和Ⅲ型胶原的生成,从而为基质的重塑奠定基础[45]。除此之外,脱细胞肌腱基质支架结构本身影响细胞的成长和迁移,它通过激活磷脂酰肌醇3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白(PI3K-AKT-mTOR)轴进一步促进肌腱干/祖细胞和其余干细胞的成腱分化过程,为组织再生和修复打下基础[31]。 α-Ⅰ型胶原、Sry相关的HMG盒基因9(Sox-9)、腱调蛋白(Tenomodulin)和聚集蛋白聚糖(Aggrecan)是评价骨髓源性干细胞成腱或成骨分化的重要因子,ROTHRAUFF等[46,49]的实验中发现向损伤部位植入支架后,腱调蛋白含量在第14天被识别到,意味着脱细胞肌腱基质支架的植入会促使骨髓源性干细胞向肌腱分化,少数为成骨分化。NING等[45]采用比格犬的跟腱作为来源制作厚度为300 μm的脱细胞肌腱切片支架,发现它能为肌腱源性干细胞的成腱分化提供微环境。这些均提示脱细胞肌腱基质支架能够维持细胞外基质内部微环境,包括细胞外基质的生化成分、原有形貌和机械行为等,其中均匀排列的胶原纤维会促使肌腱源性干细胞和骨髓源性干细胞的成腱分化。以上研究表明脱细胞肌腱基质支架的性能和环境与正常肌腱类似,促使肌腱细胞在类似的环境下增殖和迁移。 2.4.3 维持肌腱组织的生物力学性能 肌腱的机械性能与肌腱细胞密度、胶原排列和类型密切相关[48]。各种胶原类型含量的稳定对于维持肌腱细胞外基质的结构和成分、保持组织力学性能具有重要意义。肌腱细胞外基质中最主要的成分是Ⅰ型胶原和Ⅲ型胶原,损伤后的肌腱通常会引起机械性能下降和大量瘢痕组织生成,大量Ⅲ型胶原聚集在损伤部位会降低修复组织的生物力学性能[50-51]。生物力学性能的完好性对于肌腱的再生、发展和修复是必不可少的,它能诱导肌腱细胞的增殖和迁移、激活各种生长因子和蛋白激酶、激发各种生物反应等。如果在脱细胞处理过程中能保证细胞成分有效去除和细胞外基质生物力学性能保留之间的平衡,则能够维持支架一定程度的机械性能[32]。 组织细胞外基质中能够维持肌腱机械特性最为核心的是糖胺聚糖和部分生长因子[32]。脱细胞肌腱基质支架的脱细胞化处理会造成部分糖胺聚糖含量的下降,但这并不影响组织力学性能的维持,因为下降了一定程度的糖胺聚糖能为各种生长因子(转化生长因子β、碱性成纤维生长因子、胰岛素样生长因子1等)提供黏附定位点,促使组织输送多孔,促进肌腱细胞的渗透等活动,这利于组织保持其弹性和黏性[52]。脱细胞肌腱基质支架还可能通过影响干细胞的成腱分化和旁分泌过程,从而促进胶原蛋白的沉积和合成及肌腱细胞的生成,以此改善组织的生物力学性能(包括极限抗拉强度和载荷等)[48]。 WHITLOCK等[53]采用冻干人跟腱同种异体移植物应用于肌腱损伤的修复,这不仅能消除炎症反应,还扩大了支架的孔隙度,提高其拉伸特性且满足细胞的浸润化,所以脱细胞肌腱基质支架能够勉强帮助损伤后的肌腱恢复至正常肌腱的力学强度,满足临床上基本的性能要求。这提示脱细胞肌腱基质支架能通过改变支架间的孔隙率,维持细胞外基质的结构保持正常,维护肌腱细胞的生理活动,从而保护组织的生物力学性能不被破坏。尽管支架在脱细胞处理的时候会影响肌腱的最终抗拉强度,但是最终所恢复的力学性能未受较大变化。"

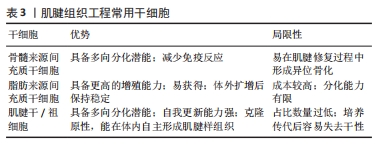
2.5 脱细胞肌腱基质支架的修饰方法 2.5.1 水凝胶 水凝胶是具有特定三维结构的水膨胀聚合物,因为它具备高亲水性、高含水量及潮湿性的特性,所以能够调节炎症反应、降低组织的感染风险、排出组织体内产生的废物和促进组织再生,是生物医学领域常见的材料[8]。经过脱细胞化处理的组织源性水凝胶支架已被广泛用于输送外源性干细胞、生长因子和其他活性因子,这些水凝胶的细胞外基质成分和原生组织类似[8,54]。由脱细胞肌腱细胞外基质组成的生物相容性水凝胶被用于促肌腱组织再生的修复支架,脱细胞化后的肌腱含有类似天然肌腱组织的细胞外基质、生物活性因子和胶原纤维等,从而促使肌腱干细胞的分化[10,55]。天然的水凝胶对于机械强度的维持能力较弱,综合脱细胞肌腱基质支架所具备的生物刚度和硬度,能更好地为干细胞转移、增殖和分化提供支撑,同时脱细胞细胞外基质肌腱水凝胶保证了细胞(成纤维细胞、转化细胞等)的多样性,这都为肌腱愈合提供理论性基础。 PARMAKSIZ等[56]利用脱细胞肌腱制备了纳米复合水凝胶支架,将细胞外基质水凝胶与具备较高糖胺聚糖和蛋白质含量的脱细胞肌腱基质支架相结合,增强此支架的力学性能及稳定性。NING等[54]从猕猴身上提取脱细胞肌腱水凝胶,制备成保留纳米纤维结构和生物活性成分的支架,促使多肢肌腱源性干细胞(mtdsc)的成腱分化和迁移活动,为肌腱再生打下基础。所以,未来脱细胞细胞外基质凝胶可能成为治疗肌腱损伤较有前景的治疗方式。 2.5.2 干细胞 近年来,脱细胞肌腱基质支架与干细胞的联合治疗在肌腱愈合过程中应用广泛,已有多位学者将支架材料和干细胞组成的复合物植入组织损伤部位以加速肌腱修复[57]。脱细胞肌腱基质支架能够作为一种载体或桥梁运输干细胞,代替损伤组织内部的异质细胞[25]。参与肌腱修复过程的干细胞主要是脂肪源性干细胞、间充质干细胞和肌腱源性干细胞,它们具备多向分化的能力,在适当机械刺激下多数能够完成腱向分化以满足肌腱修复的需求。如表3所示为肌腱组织工程常用干细胞。"

XIE等[48]的实验引入骨髓源性干细胞至“书本状”新型多层脱细胞肌腱支架修复肌腱损伤,这能够更好地将干细胞固定在支架上,避免细胞缺损、丢失、分布散乱等问题,利于肌腱的脱细胞处理和细胞的转移等活动。何树坤[57]在将肌腱源性干细胞接种到脱细胞细胞外基质支架时能够检测到基质细胞衍生因子1和单核细胞趋化蛋白的表达上升,刺激干细胞的活动。OMAE等[58]向组织损伤部位植入骨髓间充质干细胞支架后检测到高表达的基质金属肽酶13,这与细胞外基质的降解和重建密切相关,说明干细胞支架的植入能够重塑细胞外基质。上述实验均验证了干细胞联合支架的复合物对于肌腱修复有显著正向的效果,它们成功构建了一种仿生微环境,招募与组织修复相关的内源性干细胞,调动组织自身的再生能力去完成愈合过程,不过仍然需要对其深层工作机制进行研究,以找到更合适的靶点[59]。 2.5.3 静电纺丝 近年来,采取静电纺丝技术对组织脱细胞细胞外基质进行改造的方式越来越受到青睐。静电纺丝是一种多功能纳米纤维制备技术,在强电场的环境下利用聚合物溶液或熔体去除组织中的部分细胞,细胞外基质的内容和功能几乎被完全保留,广泛应用于骨科疾病的治疗当中,有望联合脱细胞肌腱基质支架支持肌腱的内源性修复[60-61]。工程师将静电纺丝技术与肌腱组织的脱细胞细胞外基质相结合,制造出具备高孔隙率和比表面积的微纤维或纳米纤维脱细胞肌腱基质支架,支架包裹住纤维,能够模拟天然肌腱组织的机械性能和细胞外基质结构,促进相关干细胞的成腱分化,这为肌腱再生提供基础[24,49,62-63]。 纳米纤维的特性影响肌腱再生。首先,纳米纤维的排列方式与肌腱细胞相关标志物(例如TNMD、关键转录因子Scleraxis和肌腱特异性转录因子Mohawk等)的表达、细胞形态变化密切相关,整齐排列的纳米纤维能够促进肌腱相关基因标志物的上调[64];其次,纳米纤维的直径影响肌腱细胞的活动,纤维的直径越小,新生成的肌腱细胞所形成的基质沉淀越多,而大直径的纤维往往会引起细胞萎缩[64]。 ZHAO等[31]首先采取静电纺丝技术制备聚乳酸纤维,通过氢气发泡技术将纤维垫转化成三维多孔支架,然后通过纤维表面改性和碳二亚胺化学将猪源性脱细胞肌腱基质移植到支架上,从而支持肌腱干/祖细胞的生理活动,同时加强细胞的黏附性。ABHARI等[61]制造了一种混合电纺丝-挤压缝线材料,能够进一步加强材料的拉伸强度并提供较低的刚度,改善缝合线的机械性能同时保留肌腱修复必须的成分。BAHRAMI等[65]制备了有不同纳米纤维排列度的静电纺聚氨酯(EPU)支架,发现这种支架能够显著支持组织内部细胞的扩散、增殖和分化活动,促使肌腱相关标志物的表达上调。总之,静电纺丝与脱细胞肌腱基质支架的联合对肌腱再生有较好的效果,未来需进一步确定纳米纤维的自身特性以更好地融入组织内部环境。 2.6 未来临床应用脱细胞肌腱基质支架的注意事项 第一,使用异体移植物时应该充分尊重物种与临床用途相一致的前提,减少疾病传播风险的同时遵守伦理守则。新鲜冷冻移植物使用率和移植成功率较高,能够显著降低疾病传播和性能低下的风险[66]。而在众多移植物的物种选择中,经过实验验证马肌腱异种移植物具备较低的人畜共患病风险,大小合适,与天然肌腱类似的力学性能等,这些优势让它成为移植物的首选物种[41,67]。 第二,有研究证实单相支架能够在腱骨愈合过程中促进纤维软骨基质和部分胶原纤维的合成与重塑,在肌腱损伤修复中使用单相支架是否是一种有效治疗手段仍然需要进一步实验验证[68]。 第三,脱细胞肌腱基质支架可以与其他人工或天然医学材料进行相融成为复合材料再植入到肌腱受损部位,这样能同时发挥各材料的特点及优势,例如静电纺丝、水凝胶、3D打印等技术,不过要确保这种“新兴材料”仍然具备较强的抗炎和抗氧化能力。 第四,实验中所构建的动物模型与人体的愈合机制、不良反应、性能等方面具有差异性,未来需要大量研究建立“金标准”评估材料的临床使用能力[69]。 第五,由于肌腱组织自身的特性:血液循环不流通,所以在制造支架的时候需要注重改善血管化,通常可以通过改造组织内的微血管系统从而加速肌腱组织的血液循环。"

| [1] 李帝均,王桂杉,刘海峰,等. 肌腱病动物模型的研究进展[J].中国实验动物学报,2022,30(2):260-266. [2] LI D, WANG G, LI J, et al. Biomaterials for Tissue-Engineered Treatment of Tendinopathy in Animal Models: A Systematic Review. Tissue Eng Part B Rev. 2023;29(4):387-413. [3] MILLAR NL, SILBERNAGEL KG, THORBORG K, et al. Tendinopathy. Nat Rev Dis Primers. 2021;7(1):1. [4] SHARMA P, MAFFULLI N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet. Neuronal Interact. 2006;6(2):181-190. [5] LUO J, WANG Z, TANG C, et al. Animal model for tendinopathy. J Orthop Translat. 2023;42:43-56. [6] KWAN KYC, NG KWK, RAO Y, et al. Effect of Aging on Tendon Biology, Biomechanics and Implications for Treatment Approaches. Int J Mol Sci. 2023;24(20):15183. [7] WANG Y, LI J. Current progress in growth factors and extracellular vesicles in tendon healing. Int Wound J. 2023;20(9):3871-3883. [8] ALKHILANI MA, HAMMOODI OT, EMRAN HA, et al. Impact of Using Processed Urinary Bladder Submucosa and Hydrogel Fabricated from Tendon on Skin Healing Process in Rabbits. Vet Med Int. 2024; 2024:6641975. [9] ALVES AN, FERNANDES KPS, DEANA AM, et al. Effects of Low-Level Laser Therapy on Skeletal Muscle Repair A Systematic Review. Am J Phys Med Rehabil. 2014;93(12):1073-1085. [10] ANJUM S, LI T, SAEED M, et al. Exploring polysaccharide and protein-enriched decellularized matrix scaffolds for tendon and ligament repair: A review. Int J Biol Macromol. 2024;254(Pt 2):127891. [11] KHAKPOUR E, TAVASSOLI A, MAHDAVI-SHAHRI N, et al. Assessing the biocompatibility of bovine tendon scaffold, a step forward in tendon tissue engineering. Cell Tissue Banking. 2023;24(1):11-24. [12] NING LJ, CUI J, HE SK, et al. Constructing a highly bioactive tendon-regenerative scaffold by surface modification of tissue-specific stem cell-derived extracellular matrix. Regen Biomater. 2022:9:rbac020. [13] MONTEIRO-LOBATO GM, RUSSO PST, WINCK FV, et al. Proteomic Analysis of Decellularized Extracellular Matrix: Achieving a Competent Biomaterial for Osteogenesis. Biomed Res Int. 2022;2022:6884370. [14] SMOAK MM, HAN A, WATSON E, et al. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng Part C Methods. 2019;25(5):276-287. [15] AL-HAKIM KHALAK F, GARCIA-VILLEN F, RUIZ-ALONSO S, et al. Decellularized Extracellular Matrix-Based Bioinks for Tendon Regeneration in Three-Dimensional Bioprinting. Int J Mol Sci. 2022;23(21):12930. [16] OTT HC, MATTHIESEN TS, GOH SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213-221. [17] OTT HC, CLIPPINGER B, CONRAD C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927-U131. [18] SONG JJ, GUYETTE JP, GILPIN SE, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646-651. [19] ZHANG X, SONG W, HAN K, et al. Three-Dimensional Bioprinting of a Structure-, Composition-, and Mechanics-Graded Biomimetic Scaffold Coated with Specific Decellularized Extracellular Matrix to Improve the Tendon-to-Bone Healing. ACS Appl Mater Interfaces. 2023;15(24):28964-28980. [20] XING S, LIU C, XU B, et al. Effects of various decellularization methods on histological and biomechanical properties of rabbit tendons. Exp Ther Med. 2014;8(2):628-634. [21] ZHANG Y, ZHANG C, LI Y, et al. Evolution of biomimetic ECM scaffolds from decellularized tissue matrix for tissue engineering: A comprehensive review. Int J Biol Macromol. 2023:246:125672. [22] CHENG CW, SOLORIO LD, ALSBERG E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32(2):462-484. [23] SANTOS ADL, SILVA CGD, BARRETO LSDS, et al. Automated Assessment of Cell Infiltration and Removal in Decellularized Scaffolds - Experimental Study in Rabbits. Rev Bras Ortop (Sao Paulo). 2021;57(6):992-1000. [24] 杨冬逸,李刚.人工肌腱/韧带生物材料研究进展[J].上海纺织科技,2024, 52(4):6-12+63. [25] WANG S, WANG Y, SONG L, et al. Decellularized tendon as a prospective scaffold for tendon repair. Mater Sci Eng C Mater Biol Appl. 2017;77:1290-1301. [26] ALBERTI KA, XU Q. Biocompatibility and degradation of tendon-derived scaffolds. Regen Biomater. 2016;3(1):1-11. [27] VEPARI C, KAPLAN DL. Silk as a biomaterial. Prog Polym Sci. 2007;32(8-9):991-1007. [28] LI J, CHEN X, HU M, et al. The application of composite scaffold materials based on decellularized vascular matrix in tissue engineering: a review. Biomed Eng Online. 2023;22(1):62. [29] IWASAKI N, ROLDO M, KARALI A, et al. In vitro development of a muscle-tendon junction construct using decellularised extracellular matrix: Effect of cyclic tensile loading. Biomater Adv. 2024:161:213873. [30] O’BRIEN FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011; 14(3):88-95. [31] ZHAO JP, ZHANG D, LAN QM, et al. Tendon Decellularized Matrix Modified Fibrous Scaffolds with Porous and Crimped Microstructure for Tendon Regeneration. Acs Applied Bio Materials. 2024;7(7):4747-4759. [32] 黄鑫.循环牵伸应力对脂肪干细胞—肌腱脱细胞支架复合物生物学特性的影响[D].武汉:华中科技大学,2024. [33] HART DA, AHMED AS, CHEN J, et al. Optimizing tendon repair and regeneration: how does the in vivo environment shape outcomes following rupture of a tendon such as the Achilles tendon? Front Bioeng Biotechnol. 2024;12:1357871. [34] NA YY, JUE H, XIA T, et al. A composite PET-matrix patch enhances tendon regeneration and tendon-to-bone integration for bridging repair of the chronic massive rotator cuff tears in a rabbit model. Regen Biomater. 2024:11:rbae061. [35] TANG CQ, CHEN YW, HUANG JY, et al. The roles of inflammatory mediators and immunocytes in tendinopathy. J Orthop Translat. 2018;14:23-33. [36] PIRES D, XAVIER M, ARAUJO T, et al. Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci. 2011;26(1):85-94. [37] MAO X, YAO L, LI M, et al. Enhancement of Tendon Repair Using Tendon-Derived Stem Cells in Small Intestinal Submucosa via M2 Macrophage Polarization. 2022;11(17):2770. [38] SOUZA NHC, MESQUITA-FERRARI RA, RODRIGUES MFSD, et al. Photobiomodulation and different macrophages phenotypes during muscle tissue repair. J Cell Mol Med. 2018;22(10):4922-4934. [39] SUNWOO JY, ELIASBERG CD, CARBALLO CB, et al. The role of the macrophage in tendinopathy and tendon healing. J Orthop Res. 2020; 38(8):1666-1675. [40] XU HT, LEE CW, LI MY, et al. The shift in macrophages polarisation after tendon injury: A systematic review. J Orthop Translat. 2020;21:24-34. [41] ZHAO LL, LUO JJ, CUI J, et al. Tannic Acid-Modified Decellularized Tendon Scaffold with Antioxidant and Anti-Inflammatory Activities for Tendon Regeneration. ACS Appl Mater Interfaces. 2024;16(13):15879-15892. [42] AMAROLI A, PASQUALE C, ZEKIY A, et al. Photobiomodulation and Oxidative Stress: 980 nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxid Med Cell Longev. 2021:2021:6626286. [43] MUSAT CL, NICULET E, CRAESCU M, et al. Pathogenesis of Musculotendinous and Fascial Injuries After Physical Exercise-Short Review. Int J Gen Med. 2023;16: 5247-5254. [44] FOK SW, GRESHAM RCH, RYAN W, et al. Macromolecular crowding and decellularization method increase the growth factor binding potential of cell-secreted extracellular matrices. Front Bioeng Biotechnol. 2023:11:1091157. [45] NING LJ, ZHANG YJ, ZHANG Y, et al. The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials. 2015;52:539-550. [46] 刘洁,周永春,惠慧.脱细胞肌腱支架对骨髓间充质干细胞增殖分化的影响[J].中国骨与关节损伤杂志,2021,36(11):1156-1159. [47] CUI J, ZHANG YJ, LI X, et al. Decellularized tendon scaffolds loaded with collagen targeted extracellular vesicles from tendon-derived stem cells facilitate tendon regeneration. J Control Release. 2023; 360:842-857. [48] XIE S, ZHOU Y, TANG Y, et al. -Book-shaped decellularized tendon matrix scaffold combined with bone marrow mesenchymal stem cells-sheets for repair of achilles tendon defect in rabbit. J Orthop Res. 2019;37(4):887-897. [49] ROTHRAUFF BB, LAURO BB, YANG G, et al. Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering. Tissue Eng Part A. 2017;23(9-10):378-389. [50] ZHAO G, ZHANG J, NIE D, et al. HMGB1 mediates the development of tendinopathy due to mechanical overloading. PLoS One. 2019;14(9):e0222369. [51] ANDARAWIS-PURI N, FLATOW EL, SOSLOWSKY LJ. Tendon Basic Science: Development, Repair, Regeneration, and Healing. J Orthop Res. 2015;33(6):780-784. [52] LANGE P, SHAH H, BIRCHALL M, et al. Characterization of a biologically derived rabbit tracheal scaffold. J Biomed Mater Res B Appl Biomater. 2017;105(7):2126-2135. [53] WHITLOCK PW, SEYLER TM, PARKS GD, et al. A Novel Process for Optimizing Musculoskeletal Allograft Tissue to Improve Safety, Ultrastructural Properties, and Cell Infiltration. J Bone Joint Surg Am. 2012;94(16):1458-1467. [54] NING LJ, ZHANG YJ, ZHANG YJ, et al. Enhancement of Migration and Tenogenic Differentiation of Macaca Mulatta Tendon-Derived Stem Cells by Decellularized Tendon Hydrogel. Front Cell Dev Biol. 2021:9:651583. [55] FARNEBO S, WOON CYL, SCHMITT T, et al. Design and Characterization of an Injectable Tendon Hydrogel: A Novel Scaffold for Guided Tissue Regeneration in the Musculoskeletal System. Tissue Eng Part A. 2014;20(9-10):1550-1561. [56] PARMAKSIZ M. Decellularized tendon-based heparinized nanocomposite scaffolds for prospective regenerative applications: Chemical, physical, thermal, mechanical and in vitro biological evaluations. J Mech Behav Biomed Mater. 2022;134:105387. [57] 何树坤.肌腱干细胞胞外基质修饰生物仿生支架促进腱骨界面愈合的实验研究[D].成都:四川大学,2021. [58] OMAE H, ZHAO C, SUN YL, et al. Multilayer Tendon Slices Seeded with Bone Marrow Stromal Cells: A Novel Composite for Tendon Engineering. J Orthop Res. 2009;27(7):937-942. [59] CUI J, NING LJ, WU FP, et al. Biomechanically and biochemically functional scaffold for recruitment of endogenous stem cells to promote tendon regeneration. NPJ Regen Med. 2022;7(1):26. [60] 吴辰,江佳慧,苏豆,等.脱细胞皮肤基质/聚氨酯混纺纤维支架促进大鼠皮肤缺损的修复[J].中国组织工程研究,2025,29(4):745-751. [61] ABHARI RE, SNELLING SJB, AUGUSTYNAK E, et al. A Hybrid Electrospun-Extruded Polydioxanone Suture for Tendon Tissue Regeneration. Tissue Eng Part A. 2024; 30(5-6):214-224. [62] 韩启斌,白浪,杨兴.生物材料改善肌腱病治疗的应用进展[J].国际骨科学杂志,2021,42(3):166-170. [63] ZHANG YM, XUE YG, REN Y, et al. Biodegradable Polymer Electrospinning for Tendon Repairment. Polymers (Basel). 2023;15(6):1566. [64] HE W, JIANG C, ZHOU P, et al. Role of tendon-derived stem cells in tendon and ligament repair: focus on tissue engineer. Front Bioeng Biotechnol. 2024:12: 1357696. [65] BAHRAMI S, SOLOUK A, DUPREZ D, et al. Microstructure Manipulation of Polyurethane-Based Macromolecular Scaffold for Tendon/Ligament Tissue Engineering. Macromol Mater Eng. 2022;307(1). doi: 10.1002/mame.202100584 [66] NETTO ADS, ANTEBI U, MORAIS CED, et al. Evaluation of Histological Properties of Human Meniscal Grafts Stored in a Tissue Bank. Rev Bras Ortop (Sao Paulo). 2020;55(6):778-782. [67] AEBERHARD PA, GROGNUZ A, PENEVEYRE C, et al. Efficient decellularization of equine tendon with preserved biomechanical properties and cytocompatibility for human tendon surgery indications. Artif Organs. 2020;44(4):E161-E171. [68] ZHANG X, HAN K, FANG Z, et al. Enhancement of Tendon-to-Bone Healing: Choose a Monophasic or Hierarchical Scaffold? Am J Sports Med. 2023;51(10):2688-2700. [69] SZCZESNY SE, CORR DT. Tendon cell and tissue culture: Perspectives and recommendations. J Orthop Res. 2023;41(10):2093-2104. [70] TAO M, LIANG F, HE J, et al. Decellularized tendon matrix membranes prevent post-surgical tendon adhesion and promote functional repair. Acta Biomater. 2021;134:160-176. [71] KOÇ-DEMIR A, ELÇIN AE, ELÇIN YM. Magnetic biocomposite scaffold based on decellularized tendon ECM and MNP-deposited halloysite nanotubes: physicochemical, thermal, rheological, mechanical andin vitrobiological evaluations. Biomed Mater. 2024;19(3). doi: 10.1088/1748-605X/ad38ab. [72] LIU Z, YU MZ, PENG H, et al. Decellularized tilapia fish skin: A novel candidate for tendon tissue engineering. Mater Today Bio. 2022:17:100488. [73] YUAN Z, CAO F, GAO C, et al. Decellularized Human Umbilical Cord Wharton Jelly Scaffold Improves Tendon Regeneration in a Rabbit Rotator Cuff Tendon Defect Model. Am J Sports Med. 2022;50(2):371-383. [74] HUANG X, LV ZT, CHENG P, et al. A Novel Low Air Pressure-Assisted Approach for the Construction of Cells-Decellularized Tendon Scaffold Complex. Curr Med Sci. 2022;42(3):569-576. [75] LOVATI AB, BOTTAGISIO M, MORETTI M. Decellularized and Engineered Tendons as Biological Substitutes: A Critical Review. Stem Cells Int. 2016:2016:7276150. [76] NAHUMI A, PEYMANI M, ASADI A, et al. Decellularized tracheal scaffold as a promising 3D scaffold for tissue engineering applications. Tissue Cell. 2023;85:102258. [77] 张珂珂,李红梅,刘畅.脱细胞随机肌腱支架促进骨髓间充质干细胞骨向分化[J].中国病理生理杂志,2020,36(4):762-768. |
| [1] | Guan Yujie, Zhao Bin. Application and prospect of artificial intelligence in screening and diagnosis of scoliosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 721-730. |
| [2] | Wang Zhipeng, Zhang Xiaogang, Zhang Hongwei, Zhao Xiyun, Li Yuanzhen, Guo Chenglong, Qin Daping, Ren Zhen. A systematic review of application value of machine learning to prognostic prediction models for patients with lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 740-748. |
| [3] | Yu Weijie, Cao Dongdong, Guo Tianci, Niu Puyu, Yang Jialin, Wang Simin, Liu Aifeng. Risk prediction models of recurrence after percutaneous endoscopic lumbar discectomy: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 749-759. |
| [4] | Tan Fengyi, Xie Jiamin, Pan Zhenfeng, Zhang Xinxu, Zheng Zetai, Zeng Zhiying, Zhou Yanfang. Effect and mechanism of collagen combined with microneedles in treatment of skin photoaging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 451-458. |
| [5] | Gu Jianmei, Yuan Kunshan, Zhou Qiang, Zhang Haijun, , . Application of laser microporous decellularized scaffolds in tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 499-507. |
| [6] | Xu Haichao, Luo Lihua, Pan Yihuai. Application and progress of dental pulp stem cells and their derivatives in dental pulp regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 153-162. |
| [7] | Liu Jing, Zhao Xiaoli, Xia Tian. Role of extracellular vesicle-mediated intercellular communication in female follicle reproduction, preimplantation embryo development and implantation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 218-228. |
| [8] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [9] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [10] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [11] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [12] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [13] | Peng Hongcheng, Peng Guoxuan, Lei Anyi, Lin Yuan, Sun Hong, Ning Xu, Shang Xianwen, Deng Jin, Huang Mingzhi . Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1497-1503. |
| [14] | Liu Haoyang, Xie Qiang, Shen Mengran, Ren Yansong, Ma Jinhui, Wang Bailiang, Yue Debo, Wang Weiguo . Application, research hotspots, and shortcomings of degradable zinc-based alloys in bone defect repair and reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 839-845. |
| [15] | Huang Haina, Yu Yanrong, Bi Jian, Huang Miao, Peng Weijie. Epigenetic characteristics of hepatogenic differentiation of mesenchymal stem cells in three-dimensional culture [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7848-7855. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||