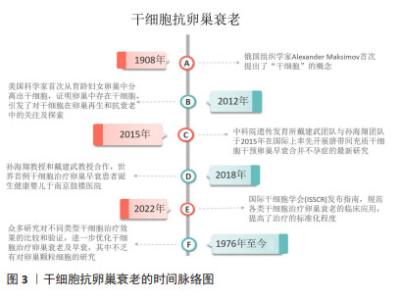
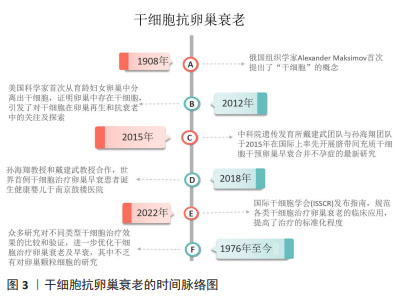
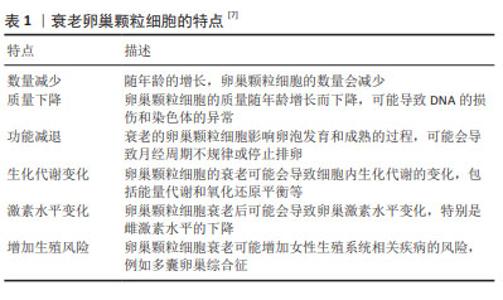
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (25): 5414-5421.doi: 10.12307/2025.521
Previous Articles Next Articles
Action mechanism and progress of stem cells against ovarian granulosa cell senescence
Lin Meiyu1, 2, 3, Zhao Xilong1, 2, 3, Gao Jing1, 2, 3, Zhao Jing1, 2, 3, Ruan Guangping1, 2, 3
- 1Basic Medical Laboratory of 920 Hospital of Joint Logistics Support Force of Chinese PLA, Kunming 650032, Yunnan Province, China; 2Integrated Engineering Research Center of Cell Biological Medicine of State and Regions, Kunming 650032, Yunnan Province, China; 3Transfer Medicine Key Laboratory of Cell Therapy Technology of Yunan Province, Kunming 650032, Yunnan Province, China
-
Received:2024-04-29Accepted:2024-06-07Online:2025-09-08Published:2024-12-26 -
Contact:Ruan Guangping, MD, Associate chief physician, Basic Medical Laboratory of 920 Hospital of Joint Logistics Support Force of Chinese PLA, Kunming 650032, Yunnan Province, China; Integrated Engineering Research Center of Cell Biological Medicine of State and Regions, Kunming 650032, Yunnan Province, China; Transfer Medicine Key Laboratory of Cell Therapy Technology of Yunan Province, Kunming 650032, Yunnan Province, China -
About author:Lin Meiyu, Master candidate, Basic Medical Laboratory of 920 Hospital of Joint Logistics Support Force of Chinese PLA, Kunming 650032, Yunnan Province, China; Integrated Engineering Research Center of Cell Biological Medicine of State and Regions, Kunming 650032, Yunnan Province, China; Transfer Medicine Key Laboratory of Cell Therapy Technology of Yunan Province, Kunming 650032, Yunnan Province, China -
Supported by:Yunnan Provincial Science and Technology Plan Major Science and Technology Project, No. 2018ZF007 (to PXH); Yunnan Provincial Key Project, No. 202101AS070039 (to RGP); 920 Hospital of Joint Logistics Support Force of Chinese PLA, No. 2020YGD12 (to RGP)
CLC Number:
Cite this article
Lin Meiyu, Zhao Xilong, Gao Jing, Zhao Jing, Ruan Guangping. Action mechanism and progress of stem cells against ovarian granulosa cell senescence[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5414-5421.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.2.1 DNA损伤和修复能力下降 DNA损伤是卵巢颗粒细胞衰老的重要驱动因素,卵巢颗粒细胞的DNA损伤日渐累积,但修复能力减弱,从而导致细胞功能失调和衰老[8]。卵母细胞和颗粒细胞因年龄增长而造成DNA损伤的诱因包括减数分裂重组、紫外线和X射线照射或者是化学物质诱导[9]。研究表明,随年龄增长,恒河猴颗粒细胞中DNA损伤增加,且DNA修复蛋白BRCA1数量减少[10],提示DNA损伤增加和修复水平降低与卵巢衰老有关[11]。在年龄增长的过程中,DNA损伤的修复机制在逐渐减弱,而无法有效及时修复DNA损伤,以致DNA损伤不断累积,使得细胞功能异常,甚至引发细胞凋亡。因此,保护DNA的完整性和提高DNA修复能力是延缓卵巢颗粒细胞衰老的策略之一。 2.2.2 氧化应激 氧化应激是卵巢颗粒细胞衰老的另外一个关键机制[12],当活性氧产生和抗氧化系统之间的平衡紊乱时,会发生氧化应激状态[13],即细胞内产生的氧自由基和其他活性氧化物超过了细胞内抗氧化防御系统的能力,这些氧化物质会与细胞的脂质、蛋白质和DNA发生反应,导致卵巢颗粒细胞中氧化应激的积累,引起了细胞膜、蛋白质和核酸的氧化损伤。大量研究表明,氧化应激对卵巢颗粒细胞的生存能力、分化潜能和免疫调节功能产生负面影响[14],氧化应激导致的细胞内氧化还原平衡的破坏可能激活细胞凋亡机制,导致颗粒细胞凋亡。 氧化应激会导致卵巢颗粒细胞线粒体功能异常[15],具体表现为线粒体内酶的活性下降、电子传递链中蛋白质的氧化损伤,进而使得线粒体内产生更多的氧自由基。研究表明,氧化应激能够通过多种途径导致卵巢颗粒细胞凋亡,例如:氧自由基直接损伤细胞膜、蛋白质和核酸[16],从而间接影响了细胞的存活能力;在细胞凋亡的信号通路中,多个分子的调控受到氧化应激直接或间接干预。针对氧化产物,卵巢颗粒细胞具有其自身的抗氧化防御系统:酶促抗氧化系统,包括超氧化物歧化酶、过氧化物酶、谷胱甘肽过氧化物酶等;还有非酶抗氧化系统。这些抗氧化防御系统可以清除氧化产物,但会因氧化产物的生成量超出抗氧化防御系统的清除极限,使得氧化产物逐渐积累[17],进一步导致颗粒细胞功能紊乱甚至凋亡[18]。表2总结了氧化应激干预卵巢颗粒细胞凋亡的主要信号通路。"


2.2.3 线粒体功能受损 随年龄增长卵巢颗粒细胞线粒体功能逐渐减弱,也可作为卵巢颗粒细胞衰老的重要机制。线粒体是很多生理过程的重要枢纽,包括能量产生、细胞氧化还原稳态和衰老。在衰老过程中线粒体自噬减少,功能失调的线粒体累积,从而影响线粒体功能[25]。线粒体是细胞内的能量生产中心,同时也是氧化应激的主要源头[26]。卵巢颗粒细胞的线粒体功能下降会导致氧化应激增加和细胞内环境的不稳定,影响细胞正常的生理功能。因衰老而受损的线粒体还会导致线粒体DNA的突变和线粒体膜电位下降,进一步影响细胞代谢、信号传导和凋亡等功能[27]。卵巢颗粒细胞衰老可能因细胞能量代谢途径异常导致线粒体功能异常,老年女性的卵巢颗粒细胞在有氧糖酵解和线粒体呼吸方面都出现下降趋势[7]。因此,维持线粒体功能的稳定性对卵巢颗粒细胞的质量和功能都尤为重要。 2.2.4 炎症反应 炎症反应是机体对外界刺激或内部损伤做出的保护性反应,可通过释放炎症递质、激活免疫细胞等方式修复损伤,然而过度的炎症反应会引发一系列问题,例如卵巢颗粒细胞的衰老。在机体衰老的过程中会伴随着慢性全身性的炎症反应,白细胞介素6、肿瘤坏死因子α等炎症递质水平升高,这种现象被称为炎性衰老[28]。细胞衰老的累积对器官损伤和疾病有重要作用,器官、生物体的衰老常常伴随着炎症反应的发生,炎症相关分子模式进一步促进细胞衰老,导致炎症进一步的发展,形成恶性循环[29],因此有研究提出炎症是加速衰老的标志[30]。细胞衰老后,会出现衰老相关分泌表型,即分泌促炎细胞因子(如白细胞介素6、白细胞介素1、S100);趋化因子(如白细胞介素8、单核细胞趋化蛋白1);可溶性受体和生长因子等[31]。炎症反应在卵巢颗粒细胞老化中起重要作用,随着年龄增长,卵巢组织中的炎症增加,导致卵巢颗粒细胞数量及功能发生改变。炎症反应的激活可能会导致颗粒细胞凋亡增加,细胞功能受损,加速其衰老进程[32]。因此,通过对衰老卵巢颗粒细胞中炎症的研究不失为一种延缓细胞衰老的策略。 2.2.5 激素水平变化 女性生殖系统在生命的不同阶段经历着激素水平的变化,如雌激素、卵泡刺激素的波动。女性体内的雌孕激素是颗粒细胞生长和发育的主要调控因子,雌激素在卵泡形成和卵巢颗粒细胞分化中发挥关键作用。随着女性年龄的增加,雌激素水平逐渐下降,这可能影响端粒酶,使得端粒显著缩短[33],进而加速细胞衰老的进程。卵泡刺激素受体主要在颗粒细胞表达,卵泡刺激素与卵泡刺激素受体的结合刺激颗粒细胞的增殖、分化,但过度的卵泡刺激素会导致颗粒细胞凋亡,卵泡刺激素还可通过抑制氧化应激诱导的线粒体自噬以促进卵巢颗粒细胞的存活[34]。因此,卵泡刺激素水平改变会影响卵巢颗粒细胞的生长发育。由此可见,激素水平的变化与卵巢颗粒细胞衰老之间存在着复杂的相互关系,激素通过调节细胞内信号通路、细胞代谢等方式,能够直接或间接影响卵巢颗粒细胞的功能和寿命。 2.3 干细胞抗卵巢颗粒细胞衰老的机制及研究进展 干细胞是一类具有自我更新和多向分化潜能特点的细胞,能够分化产生多种细胞类型,在维持自身细胞数量的同时,分化成特定类型的成熟细胞[35]。按照干细胞来源和分化潜能的不同,可将干细胞分为胚胎干细胞、成体干细胞和多能诱导干细胞。胚胎干细胞源自于早期发育的胚胎,细胞具有全能性[36];成体干细胞存在于已经发育成熟的组织中,不同于胚胎干细胞的全能性,其分化潜能较为有限[37];诱导多能干细胞是由基因工程技术将成体细胞重新编程而获得的细胞,其分化潜能类似于胚胎干细胞,可分化为多种类型的细胞[38]。干细胞疗法已经在多种疾病和损伤方面发挥重要作用,它有助于修复和代替受损组织,促进组织再生。此外,干细胞疗法已经广泛用于衰老相关疾病的治疗,干细胞自我更新和多向分化潜能的特征以及低免疫原性对干细胞抗机体衰老起到重要作用[39],干细胞疗法对延缓卵巢颗粒细胞衰老效果显著,下面将阐述治疗机制及研究进展。 2.3.1 促进增殖、抑制凋亡 干细胞治疗卵巢颗粒细胞衰老的一个重要机制是通过促进卵巢颗粒细胞的增殖、抑制凋亡来维持颗粒细胞的数量及功能。干细胞释放的生长因子和细胞因子等可以促进卵巢颗粒细胞的增殖,刺激其生长分裂,从而增加健康颗粒细胞的数量,弥补老化或损伤细胞的减少,维持卵巢颗粒细胞的稳定状态。FU等[40]用经血源性子宫内膜干细胞干预,可以有效提高卵巢颗粒细胞的活力和自噬活性,抗凋亡能力和存活率也显著增强。此外,经血源性子宫内膜干细胞通过激活磷脂酰肌醇3-激酶/蛋白激酶B(phosphoinositide 3-kinase/protein kinase B,PI3K/AKT)信号传导通路以促进卵巢颗粒细胞的增殖,提高了卵巢颗粒细胞的活力。WANG等[41]用富血小板血浆促进人脐带间充质干细胞的增殖并抑制其凋亡,从而保留了更多的干细胞用于治疗卵巢早衰大鼠。人脐带间充质干细胞和富血小板血浆的联合使用使得血清中抗苗勒管激素、雌二醇和促卵泡激素水平提高,促进了卵巢颗粒细胞的增殖。与此同时,干细胞可以通过抑制凋亡途径,有助于延缓卵巢颗粒细胞衰老的进程,促进其功能的恢复和维持。AI等[42]将脂肪干细胞静脉注射至环磷酰胺诱导的卵巢早衰大鼠体内,结果证明脂肪干细胞移植促进了抗苗勒管激素和雌二醇的分泌,抑制了卵巢颗粒细胞的凋亡。在体外实验中也证实了脂肪干细胞移植抑制了PI3K/Akt/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)轴的激活,抑制了卵巢颗粒细胞的凋亡。另外一项研究证明脐带间充质干细胞通过抗凋亡作用恢复受损卵巢颗粒细胞的功能[43]。CHEN等[44]用骨髓间充质干细胞和人胎盘间充质干细胞分别与磷酰胺芥子损伤的卵巢颗粒细胞共培养,共培养后两种干细胞均对受损卵巢颗粒细胞具有一定的修复能力,卵巢颗粒细胞的衰老和凋亡比例显著降低,且人胎盘间充质干细胞的抗衰老和抗凋亡能力略强于骨髓间充质干细胞。综上所述,干细胞作为一种新兴的治疗手段,通过促进卵巢颗粒细胞的增殖及抑制其衰老,不仅维持健康卵巢颗粒细胞的数量和功能,还延缓了卵巢颗粒细胞的衰老进程,为维持卵巢健康提供了有效途径。 2.3.2 分化潜能及再生能力 干细胞可分化成多种不同的细胞类型[36],包括卵巢颗粒细胞。干细胞向颗粒细胞定向分化,有助于修复受损组织及细胞的结构和功能,促进卵巢颗粒细胞的再生及更新,以维持卵巢正常的生理功能。在先前研究中,两种转录因子的同时过表达可以使人诱导多能干细胞分化为颗粒样细胞[45],这些转录因子可能在颗粒细胞形成过程中发挥作用。另外有研究表明,将人经血干细胞注射至卵巢早衰大鼠模型,移植2个月后追踪人经血干细胞已分化为卵巢颗粒细胞。ZHANG等[46]将大鼠胚胎干细胞和诱导多能干细胞与卵巢颗粒细胞共培养,诱导为功能性颗粒样细胞,这些细胞表达颗粒细胞的特异性受体——卵泡刺激素受体,但仍需要更多数据证明诱导分化的细胞是卵巢颗粒细胞。综上所述,干细胞的分化特性使干细胞定向分化为颗粒样细胞,表现出颗粒细胞的功能性特征,但仍需进一步研究证明诱导分化的细胞是否完全具有颗粒细胞特征。 2.3.3 分泌因子 干细胞可分泌细胞因子、外泌体和胞外囊泡等生物活性物质,这些分子可调节卵巢微环境,维持卵巢的结构和功能,对治疗卵巢颗粒细胞老化有积极作用。胰岛素样生长因子、表皮生长因子、成纤维细胞生长因子、促性腺激素和黄体生成素等都可以促进颗粒细胞增殖,抑制其凋亡,有助于修复卵巢颗粒细胞[42]。一项以脂肪干细胞治疗卵巢早衰大鼠的研究显示,脂肪干细胞可上调卵巢颗粒细胞中胰岛素样生长因子1的表达,从而增强了颗粒细胞抗凋亡的能力。肝细胞生长因子作为一种促进卵巢颗粒细胞生长分化的细胞因子,也在脂肪干细胞干预后其分泌水平显著增加[42]。 干细胞来源外泌体作为细胞间通讯的新工具,具有调节和修复受损组织的能力,能够调控卵巢颗粒细胞的功能,促进颗粒细胞生长、增殖和修复,与干细胞具有相似的功能[47]。在一项研究中,人脐带间充质干细胞来源外泌体能够促进卵巢颗粒细胞的增殖,抑制其凋亡。长链非编码RNA HCP5在人脐带间充质干细胞来源外泌体中过表达,可进一步增强外泌体的作用[48]。研究发现,人脐带间充质干细胞分泌的外泌体可增强抗炎因子白细胞介素10的表达,抑制促炎因子肿瘤坏死因子ɑ和干扰素γ的表达。另外人脐带间充质干细胞来源外泌体还具有抑制细胞凋亡和促进黄体酮生成的作用,可通过抑制核因子кB通路来改善颗粒细胞的炎症反应。除此之外,干细胞来源的细胞外囊泡对延缓卵巢颗粒细胞衰老也有积极作用,有研究证明,人脐带间充质干细胞来源细胞外囊泡对顺铂损伤的颗粒细胞具有保护作用,能够增加活细胞数量,抑制颗粒细胞凋亡,恢复卵巢颗粒细胞中类固醇激素的合成和分泌。 综上所述,干细胞及干细胞来源的细胞因子、外泌体和细胞外囊泡等物质均可通过促进颗粒细胞增殖、修复颗粒细胞损伤、维持颗粒细胞生存微环境,对治疗卵巢颗粒细胞老化具有积极作用。 2.3.4 抗氧化 抗氧化是干细胞延缓卵巢颗粒细胞衰老的一道保护屏障。干细胞释放抗氧化物质如超氧化物歧化酶,可以清除细胞内的自由基和其他氧化物质,有助于减轻卵巢颗粒细胞内的氧化应激,保护卵巢颗粒细胞维持正常结构和功能[49],延缓衰老进程。国内外有很多研究证明干细胞通过抗氧化作用延缓卵巢颗粒细胞衰老,DAI等[50]将人脐带间充质干细胞移植到卵巢早衰大鼠体内后,人脐带间充质干细胞分泌的血管内皮生长因子A通过PI3K/AKT/mTOR信号通路降低了卵巢颗粒细胞的氧化应激,缓解过度自噬,减少卵巢颗粒细胞的凋亡。另外一项研究中,移植的脂肪干细胞对卵巢自然衰老小鼠具有恢复作用,增加了卵巢颗粒细胞的增殖率。在脂肪干细胞分泌的23种生长因子中,肝细胞生长因子和碱性成纤维细胞生长因子的分泌量显著高于其他生长因子,且肝细胞生长因子和碱性成纤维细胞生长因子联合在体内和体外均可上调SIRT1/FOXO1通路,更显著抑制了氧化应激[51],从而延缓了卵巢颗粒细胞的衰老进程。这些研究有力证明了抗氧化可作为改善卵巢颗粒细胞老化的有效途径,抗氧化物质的释放可减轻卵巢颗粒细胞的氧化应激,干细胞分泌的各种生长因子也可通过调节多种信号通路减轻氧化应激,减少细胞的过度自噬和凋亡,有效延缓颗粒细胞的衰老进程。 2.3.5 抗炎症反应和免疫调节 在卵巢颗粒细胞衰老过程中,炎症反应常常被激活,导致细胞内氧化应激和细胞凋亡的发生。干细胞分泌的抗炎因子能减轻细胞内的炎症反应,以缓解卵巢颗粒细胞的炎症性衰老[52],这种抗炎作用有助于改善卵巢颗粒细胞的微环境,维持卵巢颗粒细胞的稳定性。免疫系统的异常激活或免疫应答过度会对细胞功能造成负面影响,加速卵巢颗粒细胞衰老。干细胞通过影响免疫细胞的活化及功能,从而调节免疫系统的平衡,减少免疫相关炎症和损伤以延缓卵巢颗粒细胞的衰老。DENG等[52]用人脐带间充质干细胞抑制了卵巢颗粒细胞的凋亡和炎症反应,干细胞治疗2周后显示抗炎细胞因子白细胞介素10、肿瘤坏死因子α刺激基因6和血管内皮生长因子水平显著升高,促炎细胞因子白细胞介素6、白细胞介素1β水平显著降低,从而抑制了炎症反应,改善卵巢颗粒细胞的微环境。另一研究中人脐带间充质干细胞来源外泌体具有高效的抗炎能力,人脐带间充质干细胞来源外泌体通过抑制核因子κB信号通路,增强了抗炎因子白细胞介素10的表达,抑制了促炎因子肿瘤坏死因子α和干扰素γ的表达,从而改善了卵巢颗粒细胞的炎症。炎症反应的激活是卵巢颗粒细胞衰老的一个重要特征,会导致细胞氧化应激和凋亡,加速细胞老化。干细胞疗法可有效缓解炎症反应,改善卵巢颗粒细胞微环境,进而延缓衰老进程。 表3为干细胞干预衰老卵巢颗粒细胞的机制及研究进展,图4为干细胞抗卵巢颗粒细胞衰老的机制图。"

| [1] KIRKWOOD TB. Understanding the odd science of aging. Cell. 2005; 120(4):437-447. [2] WOOD JW. Fecundity and natural fertility in humans. Oxf Rev Reprod Biol. 1989;11:61-109. [3] BROEKMANS FJ, KNAUFF EA, TE VELDE ER, et al. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18(2):58-65. [4] LIU Y, HAN M, LI X, et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum Reprod. 2017;32(12):2465-2473. [5] QIAN Y, SHAO L, YUAN C, et al. Implication of Differential Peroxiredoxin 4 Expression with Age in Ovaries of Mouse and Human for Ovarian Aging. Curr Mol Med. 2016;16(3):243-251. [6] CUKURCAM S, BETZENDAHL I, MICHEL G, et al. Influence of follicular fluid meiosis-activating sterol on aneuploidy rate and precocious chromatid segregation in aged mouse oocytes. Hum Reprod. 2007; 22(3):815-828. [7] KOBAYASHI H, YOSHIMOTO C, MATSUBARA S, et al. Altered Energy Metabolism, Mitochondrial Dysfunction, and Redox Imbalance Influencing Reproductive Performance in Granulosa Cells and Oocyte During Aging. Reprod Sci. 2024;31(4):906-916. [8] HOEIJMAKERS JH. DNA damage, aging, and cancer. N Engl J Med. 2009; 361(15):1475-1485. [9] ASHWOOD-SMITH MJ, EDWARDS RG. DNA repair by oocytes. Mol Hum Reprod. 1996;2(1):46-51. [10] ZHANG D, ZHANG X, ZENG M, et al. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet. 2015;32(7):1069-1078. [11] JOHNSON J, KEEFE DL. Ovarian aging: breaking up is hard to fix. Sci Transl Med. 2013;5(172):172fs5. [12] 崔浩亮,高锦春,史佩华,等.衰老对小鼠卵巢颗粒细胞DNA甲基化及氧化应激水平的影响[J].黑龙江畜牧兽医,2023(11): 126-130+139. [13] LIU S, JIA Y, MENG S, et al. Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. Int J Mol Sci. 2023;24(11):9205. [14] TATONE C, AMICARELLI F. The aging ovary--the poor granulosa cells. Fertil Steril. 2013;99(1):12-17. [15] OLSEN KW, CASTILLO-FERNANDEZ J, ZEDELER A, et al. A distinctive epigenetic ageing profile in human granulosa cells. Hum Reprod. 2020;35(6):1332-1345. [16] SARNIAK A, LIPIŃSKA J, TYTMAN K, et al. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postepy Hig Med Dosw (Online). 2016;70(0):1150-1165. [17] DEVINE PJ, PERREAULT SD, LUDERER U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012; 86(2):27. [18] WANG S, ZHENG Y, LI J, et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell. 2020;180(3):585-600.e19. [19] VENUGOPAL R, JAISWAL AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93(25):14960-14965. [20] ZHAO Y, PAN S, WU X. Human umbilical cord mesenchymal stem cell-derived exosomes inhibit ovarian granulosa cells inflammatory response through inhibition of NF-κB signaling in polycystic ovary syndrome. J Reprod Immunol. 2022;152:103638. [21] SAMMAD A, LUO H, HU L, et al. Transcriptome Reveals Granulosa Cells Coping through Redox, Inflammatory and Metabolic Mechanisms under Acute Heat Stress. Cells. 2022;11(9):1443. [22] DU X, LI Q, CAO Q, et al. Integrated Analysis of miRNA-mRNA Interaction Network in Porcine Granulosa Cells Undergoing Oxidative Stress. Oxid Med Cell Longev. 2019;2019:1041583. [23] SALMINEN A, KAARNIRANTA K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11(2):230-241. [24] SHIDA M, KITAJIMA Y, NAKAMURA J, et al. Impaired mitophagy activates mtROS/HIF-1α interplay and increases cancer aggressiveness in gastric cancer cells under hypoxia. Int J Oncol. 2016;48(4): 1379-1390. [25] MIWA S, KASHYAP S, CHINI E, et al. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. 2022;132(13):e158447. [26] ABATE M, FESTA A, FALCO M, et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin Cell Dev Biol. 2020;98: 139-153. [27] GUO Y, GUAN T, SHAFIQ K, et al. Mitochondrial dysfunction in aging. Ageing Res Rev. 2023;88:101955. [28] FRANCESCHI C, BONAFÈ M, VALENSIN S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244-254. [29] LI X, LI C, ZHANG W, et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239. [30] FERRUCCI L, FABBRI E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9): 505-522. [31] TEISSIER T, BOULANGER E, COX LS. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells. 2022; 11(3):359. [32] CAMAIONI A, UCCI MA, CAMPAGNOLO L, et al. The process of ovarian aging: it is not just about oocytes and granulosa cells. J Assist Reprod Genet. 2022;39(4):783-792. [33] BAYNE S, LI H, JONES ME, et al. Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell. 2011;2(4):333-346. [34] SHEN M, JIANG Y, GUAN Z, et al. FSH protects mouse granulosa cells from oxidative damage by repressing mitophagy. Sci Rep. 2016;6: 38090. [35] JIN J. Stem Cell Treatments. JAMA. 2017;317(3):330. [36] ZAKRZEWSKI W, DOBRZYŃSKI M, SZYMONOWICZ M, et al. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. [37] CAPLAN AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650. [38] TAKAHASHI K, YAMANAKA S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. [39] HWANG JJ, RIM YA, NAM Y, et al. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front Immunol. 2021;12: 631291. [40] FU X, ZHANG S, LI T, et al. Menstrual blood-derived endometrial stem cells ameliorate the viability of ovarian granulosa cells injured by cisplatin through activating autophagy. Reprod Toxicol. 2022;110:39-48. [41] WANG J, ZHAO Y, ZHENG F, et al. Activated Human Umbilical Cord Blood Platelet-Rich Plasma Enhances the Beneficial Effects of Human Umbilical Cord Mesenchymal Stem Cells in Chemotherapy-Induced POF Rats. Stem Cells Int. 2021;2021:8293699. [42] AI G, MENG M, GUO J, et al. Adipose-derived stem cells promote the repair of chemotherapy-induced premature ovarian failure by inhibiting granulosa cells apoptosis and senescence. Stem Cell Res Ther. 2023;14(1):75. [43] SEOK J, PARK HS, CETIN E, et al. The potent paracrine effect of umbilical cord mesenchymal stem cells mediates mitochondrial quality control to restore chemotherapy-induced damage in ovarian granulosa cells. Biomed Pharmacother. 2024;172:116263. [44] CHEN S, WANG Y, LIAO L, et al. Similar Repair Effects of Human Placenta, Bone Marrow Mesenchymal Stem Cells, and Their Exosomes for Damaged SVOG Ovarian Granulosa Cells. Stem Cells Int. 2020; 2020:8861557. [45] PIERSON SMELA MD, KRAMME CC, FORTUNA PRJ, et al. Directed differentiation of human iPSCs to functional ovarian granulosa-like cells via transcription factor overexpression. Elife. 2023;12:e83291. [46] ZHANG J, LI H, WU Z, et al. Differentiation of rat iPS cells and ES cells into granulosa cell-like cells in vitro. Acta Biochim Biophys Sin (Shanghai). 2013;45(4):289-295. [47] SUN B, MA Y, WANG F, et al. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther. 2019;10(1):360. [48] SUN YT, CAI JH, BAO S. Overexpression of lncRNA HCP5 in human umbilical cord mesenchymal stem cell-derived exosomes promoted the proliferation and inhibited the apoptosis of ovarian granulosa cells via the musashi RNA-binding protein 2/oestrogen receptor alpha 1 axis. Endocr J. 2022;69(9):1117-1129. [49] STAVELY R, NURGALI K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. 2020;9(9): 985-1006. [50] DAI W, YANG H, XU B, et al. Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) alleviate excessive autophagy of ovarian granular cells through VEGFA/PI3K/AKT/mTOR pathway in premature ovarian failure rat model. J Ovarian Res. 2023;16(1):198. [51] DING C, ZOU Q, WANG F, et al. HGF and BFGF Secretion by Human Adipose-Derived Stem Cells Improves Ovarian Function During Natural Aging via Activation of the SIRT1/FOXO1 Signaling Pathway. Cell Physiol Biochem. 2018;45(4):1316-1332. [52] DENG T, HE J, YAO Q, et al. Human Umbilical Cord Mesenchymal Stem Cells Improve Ovarian Function in Chemotherapy-Induced Premature Ovarian Failure Mice Through Inhibiting Apoptosis and Inflammation via a Paracrine Mechanism. Reprod Sci. 2021;28(6):1718-1732. [53] DWININGSIH SR, DARMOSOEKARTO S, HENDARTO H, et al. Effects of bone marrow mesenchymal stem cell transplantation on tumor necrosis factor-alpha receptor 1 expression, granulosa cell apoptosis, and folliculogenesis repair in endometriosis mouse models. Vet World. 2021;14(7):1788-1796. [54] NOORY P, NAVID S, ZANGANEH BM, et al. Human Menstrual Blood Stem Cell-Derived Granulosa Cells Participate in Ovarian Follicle Formation in a Rat Model of Premature Ovarian Failure In Vivo. Cell Reprogram. 2019;21(5):249-259. [55] LI H, ZHAO W, WANG L, et al. Human placenta-derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by IRE1α pathway in autoimmune POF mice. Cell Biol Int. 2019;43(8):899-909. [56] QU Q, LIU L, CUI Y, et al. miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res Ther. 2022;13(1):352. [57] GAO T, CHEN Y, HU M, et al. MicroRNA-22-3p in human umbilical cord mesenchymal stem cell-secreted exosomes inhibits granulosa cell apoptosis by targeting KLF6 and ATF4-ATF3-CHOP pathway in POF mice. Apoptosis. 2023;28(7-8):997-1011. [58] IZADI M, REZVANI ME, ALIABADI A, et al. Mesenchymal stem cells-derived exosomes as a promising new approach for the treatment of infertility caused by polycystic ovary syndrome. Front Pharmacol. 2022;13:1021581. [59] SUN L, LI D, SONG K, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci Rep. 2017; 7(1):2552. [60] ZHANG J, YIN H, JIANG H, et al. The protective effects of human umbilical cord mesenchymal stem cell-derived extracellular vesicles on cisplatin-damaged granulosa cells. Taiwan J Obstet Gynecol. 2020; 59(4):527-533. [61] LI N, FAN X, LIU L, et al. Therapeutic effects of human umbilical cord mesenchymal stem cell-derived extracellular vesicles on ovarian functions through the PI3K/Akt cascade in mice with premature ovarian failure. Eur J Histochem. 2023;67(3):3506. [62] GAO T, CAO Y, HU M, et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MicroRNA-29a Improves Ovarian Function of Mice with Primary Ovarian Insufficiency by Targeting HMG-Box Transcription Factor/Wnt/β-Catenin Signaling. Dis Markers. 2022;2022:5045873. [63] PU X, ZHANG L, ZHANG P, et al. Human UC-MSC-derived exosomes facilitate ovarian renovation in rats with chemotherapy-induced premature ovarian insufficiency. Front Endocrinol (Lausanne). 2023; 14:1205901. [64] ZHOU Y, ZHOU J, XU X, et al. Matrigel/Umbilical Cord-Derived Mesenchymal Stem Cells Promote Granulosa Cell Proliferation and Ovarian Vascularization in a Mouse Model of Premature Ovarian Failure. Stem Cells Dev. 2021;30(15):782-796. [65] CHANG L, FAN W, PAN X, Zhu X. Stem cells to reverse aging. Chin Med J (Engl). 2022;135(8):901-910. [66] LO KC, CHUANG WW, LAMB DJ. Stem cell research: the facts, the myths and the promises. J Urol. 2003;170(6 Pt 1):2453-2458. [67] 李仲康,郑嘉华,田彦鹏,等.间充质干细胞治疗卵巢早衰的最新进展及机制[J].中国组织工程研究,2022,26(1):141-147. |
| [1] | Miao Jiahang, Ma Sheng, Li Qupeng, Yu Huilin, Hu Tianyu, Gao Xiao, Feng Hu. Cervical lordosis ratio can be used as a decision-making indicator for selection of posterior surgical approach for multi-level cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1796-1802. |
| [2] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [3] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [4] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [5] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [6] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [7] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [8] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [9] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [10] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [11] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [12] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [13] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [14] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [15] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||