Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (22): 4795-4803.doi: 10.12307/2025.466
Previous Articles Next Articles
Effects of self-assembling peptide hydrogels on tumor treatment
He Huanhuan1, 2, Tian Shijia1, 2, Chen Luoying1, 2, Liu Yanfei1, 2
- 1Key Laboratory of Cell Engineering, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2Organizational Injury Repair and Regenerative Medicine Provincial and Departmental Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2024-06-22Accepted:2024-07-27Online:2025-08-08Published:2024-12-06 -
Contact:Liu Yanfei, Associate professor, Key Laboratory of Cell Engineering, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Organizational Injury Repair and Regenerative Medicine Provincial and Departmental Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:He Huanhuan, Master candidate, Key Laboratory of Cell Engineering, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Organizational Injury Repair and Regenerative Medicine Provincial and Departmental Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:Basic Research Project of Guizhou Provincial Department of Science and Technology, No. ZK[2024]316 (to LYF)
CLC Number:
Cite this article
He Huanhuan, Tian Shijia, Chen Luoying, Liu Yanfei. Effects of self-assembling peptide hydrogels on tumor treatment[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4795-4803.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
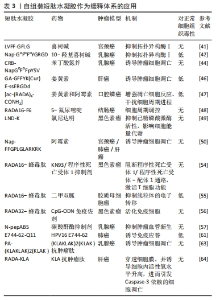
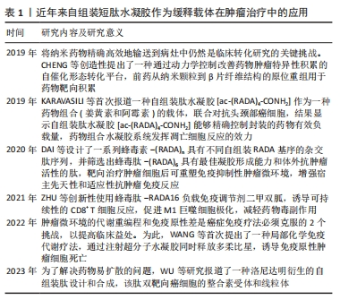
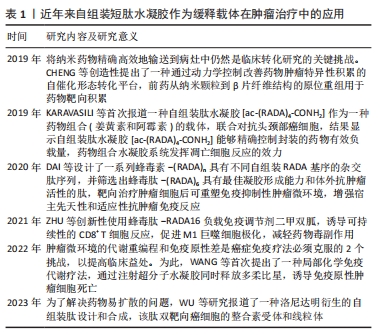
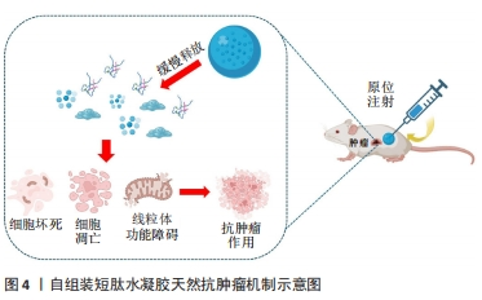
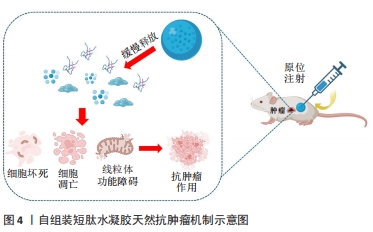
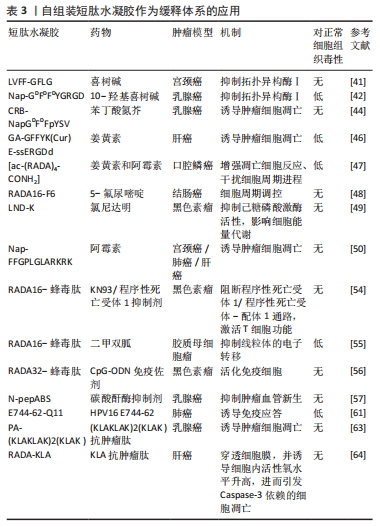
2.2 短肽自组装的机制 短肽自组装是多种作用力协同合作的结果,包括氢键、π-π堆叠、疏水效应、范德华力和静电相互作用等[24]。值得注意的是,在生物医学中,水通常是自组装的介质,由于水分子的参与,水相中氢键和静电相互作用的能量贡献与气相有很大不同[25]。DU等[26]认为自组装中的氢键和静电相互作用是短肽自组装贡献最大的作用力,静电相互作用既具有排斥性又具有吸引性,而其他4种非共价键在相似的键能水平上提供吸引相互作用。 2.2.1 氢键 聚酰胺主链以及极性和带电原子酸残基提供了丰富的氢键给体和受体,这使得氢键成为肽自组装中的主要相互作用[25]。分子内和分子间的氢键分别促进二级结构α-螺旋和β-折叠的形成[27]。 2.2.2 π-π堆叠 π-π堆叠是构建纳米结构的重要相互作用力[28]。由于π-π堆叠作用存在,自组装有利于沿垂直于芳香平面的方向生长。LAMPEL等[29]提出,短肽自组装的中若有2个芳香族则有利于三肽的自组装,在二肽和三肽中引入9-芴基甲基氧羰基(Fmoc)基团来促进自组装并实现有序的纳米结构。 2.2.3 疏水效应 疏水效应是指疏水基团(如极性残基和脂肪尾部)在水中聚集的趋势,这是蛋白质折叠的主要作用之一[30]。疏水相互作用表现出强烈的温度依赖性,在60 ℃以下疏水相互作用随着温度的降低而降低。 2.2.4 静电相互作用 静电相互作用对于调节疏水和亲水之间界面的曲率非常重要。在pH值为2的条件下,Ac-KLVFFA自组装形成纳米带,而无帽KLVFFA肽在N端携带一个正电荷,β-折叠的存在促使短肽自组装形成纳米纤维[31]。由于-COOH和-NH2基团的弱酸和弱碱性质,静电相互作用可以很容易地通过pH值进行调节[32];同时,溶液中的盐效应屏蔽了静电相互作用,盐浓度和离子也会影响静电相互作用,最终影响短肽的自组装[33]。 2.3 自组装短肽水凝胶作为缓释递送载体在肿瘤治疗中的应用 抗肿瘤药物与短肽纤维水凝胶之间的相互作用是实现药物分子可控释放的关键因素之一。一般来讲,游离的药物成分可通过与短肽溶液进行简单的混合,在凝胶形成之后即可完成药物的包埋。但是由于小分子药物的大小通常远小于短肽水凝胶的孔径,因此,药物的扩散(释放)速度和与二者类型和相互作用紧密相关,而二者的相互作用由药物分子和短肽分子的化学性质所决定。肿瘤治疗药物通常是复杂的分子,可以产生多种分子相互作用,比如大部分药物化合物都含有芳香基团,这些基团能通过诸如阳离子-π和π-π作用等参与生物识别过程。因此,选择性引入特定氨基酸可赋予自我组装肽特定的相互作用能力,如氢键、亲水/疏水、静电、阳离子-π和π-π作用。通过短肽分子[VEVKVEVK (VEK2)、VKVKVEVK(VEK3)和VEVEVKVE (VEK1)]表面电荷能够调控带电药物的扩散速度[34-35]。F8短肽凝胶可通过阳离子-π和π-π作用调节阿霉素的释放。此外,还可以设计非天然氨基酸来引入特定的功能和相互作用能力[36]。 此外,也可以将药物成分或者药物前体直接共价连接在短肽分子上,以此产生包含药物成分的新型药物/短肽复合水凝胶,这种复合凝胶能够有效提高小分子药物的溶解度、稳定度,同时大幅延长药物的释放时间,通过增强通透性和滞留效应提高药物在肿瘤区域的积累。自组装短肽水凝胶作为缓释递送载体在肿瘤治疗中展现出良好的应用前景。根据不同类型的药物应用,该文将其分为以下几个方面进行详细描述。 2.3.1 小分子抗肿瘤药物 小分子抗肿瘤药物是指分子质量较小、能够渗透细胞膜并影响肿瘤细胞生长或代谢的药物,它们通常通过靶向特定的分子靶点来抑制肿瘤细胞的增殖或诱导其凋亡[37]。喜树碱、苯丁酸氮芥、5-氟尿嘧啶等可以特异性地阻断肿瘤的增殖和生长过程中的信号传导通路,起到抗肿瘤的作用[38]。 喜树碱属于一类拓扑异构酶Ⅰ抑制剂,主要用于治疗某些实体肿瘤如结直肠癌和卵巢癌等,它通过阻断DNA拓扑异构酶Ⅰ的作用抑制DNA的超螺旋化,导致肿瘤细胞DNA的断裂和凋亡[39-40]。为了减轻喜树碱的毒副作用,增强其在病患部位的治疗作用,CHENG等[41]设计了具有β-折叠纤维支架结构的喜树碱短肽前药,负载药物的短肽达到肿瘤部位后亲水性外壳脱落并分解,快速地从纳米颗粒转变成纳米纤维,使抗肿瘤药物喜树碱得以持续释放并滞留于肿瘤病灶范围,从而提高治疗的效果,减少药物的毒副作用。LIU等[42]设计并合成了Nap-GFFYGRGD和Nap-GDFDFDYGRGD(分别为L-肽和D-肽)负载10-羟基喜树碱的自组装肽水凝胶,短肽中的RGD基序一方面增加肽的亲水性,以达到自组装的目的,另一方面赋予自组装纳米纤维靶向肿瘤能力;在这项研究中,L-肽和D-肽纳米纤维包封10-羟基喜树碱后,10-羟基喜树碱在水中的溶解度分别提高了51倍和46倍,纳米纤维通过增强小分子抗肿瘤药物对肿瘤组织的渗透性和滞留性效应靶向递送,显著抑制乳腺癌(4T1细胞)荷瘤小鼠肿瘤生长。 苯丁酸氮芥是一种氮芥类化学药物,属于碱化剂类抗肿瘤药物的一种,它能够与DNA分子中的氨基基团发生亲核反应,导致DNA的碱基修复障碍和交联,从而阻断肿瘤细胞的DNA复制和转录过程,最终诱导肿瘤细胞凋亡[43]。LIANG等[44]设计的苯丁酸氮芥-GDFDFpDY短肽呈α-螺旋结构,通过持续释放苯丁酸氮芥前体物质NapGDFDFpYSV,肿瘤部位过表达的碱性磷酸酶切割苯丁酸氮芥-NapGDFDFpYSV短肽并原位自组装形成水凝胶缓释苯丁酸氮芥释放,从而提高了肿瘤细胞的摄取,增强对肿瘤细胞的杀伤作用。 姜黄素是一种亲脂性多酚抑制剂,具有明显抑制肝癌细胞生长的作用,然而较差的水溶性、生物利用度和药代动力学特征限制了姜黄素的治疗用途[45]。CHEN等[46]将GFFYK自组装序列通过二硫键和亲水序列 ERGD包裹姜黄素,构建GA-GFFYK(Cur)E-ssERGDd自组装短肽水凝胶,用于肝癌组织中谷胱甘肽识别并还原二硫键,姜黄素可以通过酯键水解从凝胶中释放出来,进一步提高凝胶对肿瘤细胞的抑制能力。药物的水溶性是药物释放速率的决定因素。KARAVASILI等[47]报道了一种自组装肽水凝胶[ac-(RADA)4-CONH2]作为一种药物组合(姜黄素和阿霉素)的载体,用于对抗头颈部癌细胞,结果显示自组装肽水凝胶[ac-(RADA)4-CONH2]能够精确控制封装的药物有效负载量,药物组合水凝胶系统发挥凋亡细胞反应的效力;动物实验结果显示该自组装肽水凝胶可以作为局部联合治疗头颈部癌症的有利候选药物载体。 还有一些其他小分子抗肿瘤药物的缓释,如ASHWAVIKLVNAR等[48]发现具有pH值响应性自组装短肽RADA-F6可以有效地用作药物递送系统,在碱性pH值下可持续释放小分子抗肿瘤药物5-氟尿嘧啶;由于5-氟尿嘧啶含有芳香族嘧啶环,RADA-F6体系与苯丙氨酸有效π-π堆积而捕获芳香族药物,能够表现出更好的控释行为;以RADA16为对照的分子动力学模拟证实了RADA-F6在不同条件下的稳定性和可控释放特性,证实RADA-F6 可成功用作pH值敏感、受控5-氟尿嘧啶递送系统的有效载体。WU等[49]设计自组装短肽 LND-K负载小分子抗肿瘤药物氯尼达明,不仅能够延缓氯尼达明的释放,降低肿瘤细胞生长活性,而且诱导肿瘤细胞内三磷酸腺苷和线粒体膜电位水平下降,联合光动力疗法抗肿瘤疗效更为明显。CAO等[50]设计的具有疏水内核的长纤维短肽分子Nap-FFGPLGLARKRK能够作为缓释载体包埋阿霉素,其中Nap-FF是组装序列,GPLGLA是基质金属蛋白酶7的敏感位点,RKRK可促进与细胞膜的相互作用;肿瘤组织过表达的基质金属蛋白酶7能够水解该凝胶,从而持续释放阿霉素,实现肿瘤靶向药物传递和对肿瘤细胞的选择性杀伤;小鼠体内抗肿瘤实验证实了这种酶敏感肽药物载体在成功抑制肿瘤生长和转移的同时,显著减少不良反应的高效性。 2.3.2 免疫治疗药物 免疫治疗药物是一类利用免疫系统来治疗疾病的药物,主要包括免疫调节剂、细胞治疗药物等[51]。免疫治疗药物的作用特点是通过激活或抑制免疫系统的功能帮助机体识别并攻击异物或异常细胞,具有较强的特异性和长期的抗瘤效应[52],在治疗癌症、自身免疫性疾病等方面取得了一定的疗效,然而治疗过程中通常伴有较为明显的免疫相关不良反应[53]。 钙离子/钙调蛋白依赖激酶Ⅱ抑制剂KN93只能诱导小部分CD8+ T细胞介导的抗肿瘤免疫反应。DAI等[54]用蜂毒肽修饰RADA16形成短肽MR52水凝胶,再包封KN93分子形成MRK复合水凝胶,从而提高 KN93肿瘤细胞摄取效率,重塑肿瘤微环境,交替活化的M2型肿瘤相关巨噬细胞(M2-TAM)和M1型肿瘤相关巨噬细胞(M1-TAM);局部持续缓释KN93能够显著提高免疫刺激水平,最大限度地发挥其直接抗肿瘤和免疫调节作用;进一步将MRK水凝胶负载程序性死亡受体1抗体,可对黑色素瘤提供更有效的治疗效果,治疗效果提高30%。ZHU等[55]基于蜂毒肽-RADA16负载免疫调节剂二甲双胍,诱导可持续性的CD8+ T细胞反应,促进M1巨噬细胞极化,调节缺氧诱导因子1α、程序性死亡受体-配体1的表达,进而增强了适应性免疫系统对肿瘤发生和发展的抑制作用,在体内外都表现出了对胶质母细胞瘤的杀伤作用。YANG等[56]将蜂毒肽和自组装短肽RADA32组装形成MR水凝胶,将免疫佐剂CpG-ODN包封到MR水凝胶中形成可注射的MCL水凝胶,进而激活树突状细胞和细胞毒性T淋巴细胞直接杀伤黑色素瘤细胞,抑制荷瘤小鼠的肿瘤生长。碳酸酐酶Ⅸ被称为肿瘤相关酶,仅在肿瘤的缺氧区域过表达。LI等[57]设计载体型氨基酸短肽N-pepABS负载碳酸酐酶抑制剂,将碳酸酐酶抑制剂定位在低氧癌细胞表面并增强其抑制效力和选择性;碳酸酐酶Ⅸ相关的胞吞作用也促进了缺氧条件下短肽纳米纤维的选择性细胞内摄取,其中纳米纤维结构随着pH值的降低而增大,这种效应导致细胞内酸囊泡损伤并阻断保护性自噬;在乳腺肿瘤体内实验证明了N-pepABS负载碳酸酐酶抑制剂具有更强的抗转移和抗血管生成作用。 在抗肿瘤免疫的各种免疫抑制代谢物中,色氨酸分解代谢物犬尿氨酸是一个有吸引力的阻断靶点。WANG等[58]提出了一种局部化学免疫代谢疗法,通过注射超分子短肽水凝胶同时释放多柔比星,诱导免疫原性肿瘤细胞死亡和犬尿烯酶,破坏肿瘤微环境中犬尿氨酸介导的免疫抑制途径;超分子短肽水凝胶KTNase协同免疫治疗药物增强肿瘤免疫原性并释放抗肿瘤免疫力,在三阴性乳腺癌和黑色素瘤的小鼠模型中,单次低剂量肿瘤周围注射治疗性水凝胶可促进肿瘤微环境向更具有免疫刺激性的转变,从而增强肿瘤抑制并延长小鼠存活期;此外,局部治疗诱导的全身抗肿瘤效应可防止切除后肿瘤复发。这种短肽水凝胶负载免疫治疗药物可作为增强抗肿瘤免疫力和提高癌症免疫疗法疗效的一般策略。 细胞表面组织相容性复合体Ⅰ类表达的下调是肿瘤细胞逃避T细胞介导的抗肿瘤免疫的常见机制,这种下调是阻碍抗肿瘤疫苗功效的障碍之一[59-60]。故此,将自组装短肽水凝胶对抗原抗体进行特定位点的修饰,提高抗原抗体的免疫原性,进而提高抗肿瘤的效果。REINIS等[61]研究了自组装短肽和树突状细胞的疫苗在组织相容性复合体Ⅰ类缺陷肿瘤(TC-1/A9)小鼠模型中的疗效,将能够识别细胞毒性T淋巴细胞的HPV16 E744-62抗原肽连接在短肽Q11氨基末端,在磷酸盐缓冲溶液中自组装形成纳米纤维E744-62-Q11,同时负载和比较了两种 CpG ODN(CpG ODN 1826和CpG ODN 1585)的佐剂疗效,结果显示,与CpG ODN 1826相比,联合CpG ODN 1585更有效地抑制了TC-1/A9肿瘤的生长。 2.3.3 抗肿瘤序列 抗肿瘤肽是一类具有抑制细胞分裂、抗肿瘤的短肽。目前研究较为广泛的蜂毒肽(GIGAVLKVLTGLPALISWIKRQQ)是蜂毒的主要成分,是目前人类所知抗炎性最强的活性物质之一。蜂毒肽作为单体在中性水溶液中以随机卷曲结构存在,随着pH值以及离子强度的增高,蜂毒肽自我交联形成螺旋四聚体结构,该四聚体结构中的螺旋表面形成具有极性面和非极性面的两亲分子,这种特性使得蜂毒肽能够嵌入和破坏磷脂双分子层,从而抑制肿瘤细胞生长发育。然而,蜂毒肽具有强烈的溶血活性,对正常细胞也有较强的毒性。YANG等[56]将抗肿瘤序列蜂毒肽和自组装短肽RADA32组装形成水凝胶骨架Melittin-RADA32(MR),随后将全肿瘤细胞裂解物和免疫佐剂CpG-ODN负载到MR中,以开发可注射且具有细胞毒性的水凝胶MCL;在体内实验中,MCL不仅表现出优异的持续药物释放能力,发挥直接的抗肿瘤活性,而且具有强大的免疫起始作用,激活引流淋巴结中的树突状细胞和肿瘤微环境中细胞毒性T淋巴细胞的浸润;动物实验证实,MCL可以有效抑制B16F10荷瘤小鼠的肿瘤生长,表明MCL水凝胶是治疗黑色素瘤的潜在癌症疫苗策略。抗肿瘤阳离子肽KLA(KLAKLAKLAK)是一种促凋亡肽,当细胞内化时可以通过破坏线粒体膜来诱导肿瘤细胞快速凋亡。为了增强KLA肽抗肿瘤作用,HAO等[62]将KLA肽与细胞渗透肽TAT化学偶联,并与阳离子活性肽HPRP-A1共给药,通过体外实验验证了KLA-TAT肽和HPRP-A1的双重抗肿瘤机制——诱导细胞膜破坏和细胞凋亡。然而抗肿瘤肽KLA在体内易降解,为此,STEPHANY等[63]将阳离子α-螺旋KLA肽整合到两亲性肽PA中,自组装成生物活性圆柱形纳米纤维结构短肽水凝胶,通过裂解细胞膜介导乳腺癌细胞非依赖性天冬氨酸蛋白水解酶死亡,选择性抑制肿瘤细胞的生长,显著增加肿瘤细胞对KLA序列的摄取;相比传统纳米颗粒载体,PA 纳米纤维在体内的循环时间更长,影响癌细胞的存活、黏附和迁移,并且PA是可生物降解的载体,可分解成氨基酸和脂质,对生物体无害。LIU等[64]合成了一种由48个氨基酸组成的短肽RADA-KLA,它可以自组装成具有抗肿瘤活性的纳米纤维结构,研究进一步证明了RADA-KLA自组装短肽可以诱导肝癌细胞死亡并抑制其黏附和迁移,随着RADA-KLA浓度的增加抑制作用增强。 自组装短肽水凝胶作为缓释体系的应用,见表3。"

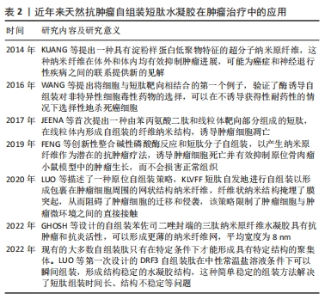
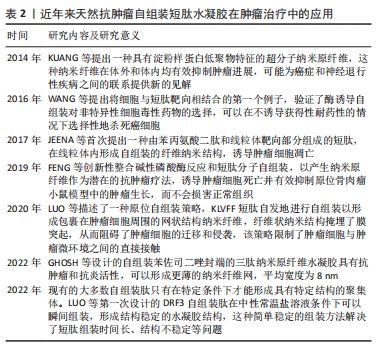
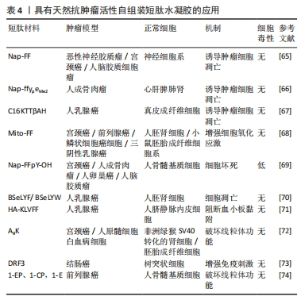
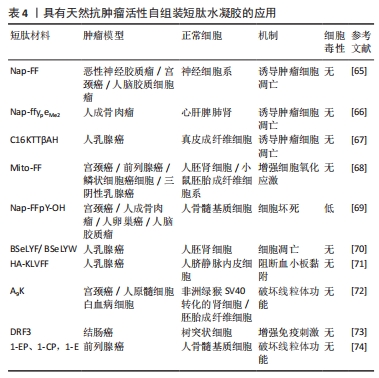
KUANG等[65]发现,在β-淀粉样蛋白中的二肽片段转化为超分子水凝胶的过程中,由二苯丙氨酸[淀粉样蛋白(Aβ)的核心基序]和萘组成的自组装肽Nap-FF抑制肿瘤生长,苯基和萘基的加入增强了芳香族-芳香族相互作用,促进了水中分子自组装,形成纳米纤维结构;虽然Nap-FF的单体是无害的,但其形成的纳米纤维可以选择性地抑制胶质母细胞瘤细胞(T78G)在神经元细胞上的生长,并且在Hela小鼠模型中能有效抑制肿瘤细胞生长。这项工作表明源自淀粉样蛋白核心基序的纳米纤维是抑制肿瘤进展的有效药物。FENG等[66]开发了一种由2部分组成的前体,亲水嵌段(即磷酸酪氨酸)连接的两亲性自组装肽Nap-ffypeMe2,碱性磷酸酶在骨肉瘤癌细胞(例如 Saos-2)上和骨肉瘤癌细胞中过表达,可从前体的酪氨酸残基中切割磷酸盐并触发短肽自组装,Nap-ffypeMe2(1P)在肿瘤细胞内部选择性转变成Nap-ffyeMe2,诱导肿瘤细胞死亡并有效抑制原位骨肉瘤小鼠模型中的肿瘤生长,使肿瘤体积减少25倍,而不会损害正常器官。CASTELLETTO等[67]发现自组装短肽C16-β-丙氨酸-组氨酸含有生物活性物质β-丙氨酸-组氨酸基序,这个基序广泛存在于体内,具有抗氧化等特性,随后将KTT肽序列与脂肽C16-β-丙氨酸-组氨酸共组装形成具有β-折叠结构的短肽C16KTTβ-丙氨酸-组氨酸,形成延伸类似于细胞外基质的纳米纤维结构网络结构,选择性地杀死乳腺癌细胞(MCF-7),减少对正常细胞的毒性。 Mito-FF是一种特殊的肽两亲物,具有线粒体靶向部分的苯丙氨酸二肽,也称为三苯基膦,这种分子在活细胞内能够实现分子自组装,与生物功能的激活有关。 Mito-FF优先积聚在线粒体内,并达到临界聚集浓度,从而形成纳米纤维结构。Mito-FF纳米纤维结构能够诱导线粒体功能障碍,通过膜破坏引发细胞凋亡,因此,Mito-FF超分子结构为治疗肿瘤和深入研究细胞功能提供了新的视角。JEENA等[68]进一步发现,由于负膜电位高,Mito-FF在癌细胞线粒体中积累,浓度增加导致Mito-FF自组装成纳米纤维结构;在正常细胞中未能观察到纳米纤维形成,在肿瘤细胞人前列腺癌(PC3)、宫颈癌(HeLa)、人结肠癌 (HT-29)中Mito-FF 纳米纤维结构破坏了线粒体膜并激活了针对肿瘤细胞的内在凋亡途径,抑制肿瘤细胞的生长。 三苯基膦是一种能够特异性靶向线粒体的有机磷化合物。目前研究发现,将生物活性分子或治疗药物和三苯基膦进行连接能够增强药物对肿瘤的靶向和抗肿瘤活性,减小药物的多药耐药性产生概率。WANG等[69]将三苯基膦与一个四肽自组装基序(FFYK)结合,形成的水凝胶能够选择性靶向并杀死Saos2细胞而不引起耐药性,并减轻药物对人骨髓基质(HS5)细胞的毒性。GHOSH等[70]设计的苯并硒二唑(Bse)修饰的LYF(L= L-亮氨酸;Y= L-酪氨酸;F=L-苯丙氨酸)和 BSeLYW(W=L-色氨酸)三肽水凝胶,BseLYF形成更薄的纳米纤维网,平均宽度为8 nm,而BSeLYW水凝胶形成了更密集的3D纳米纤维网络,通过激活Bax、CytC、Apaf和Caspase-9诱导活性氧过量产生,激活内在凋亡途径,剂量依赖性抑制MCF-7细胞的生长,并且在较低的浓度下对人肾脏胚胎细胞(HEK 293T)无毒性。LUO等[71]将KLVFF短肽基序通过透明质酸功能化脂质体靶向肿瘤组织并自发地自组装形成纳米纤维,呈网状结构包裹在肿瘤细胞周围,阻断肿瘤细胞诱导的体外血小板聚集和黏附,限制荷瘤小鼠模型的肿瘤生长和转移,表明通过调节肿瘤细胞与肿瘤微环境之间的相互作用有助于抑制肿瘤的进展和转移。 XU等[72]报道A9K短肽可显著抑制HeLa细胞和人早幼粒细胞白血病HL60细胞生长,同时对成纤维细胞(Cos 7)、小鼠成纤维细胞(NIH3T3)具有低毒作用;进一步的研究表明,A9K能够穿透肿瘤细胞膜并破坏膜结构造成细胞坏死,引起线粒体功能障碍,导致Bcl-2和c-myc基因的转录下调,发生线粒体凋亡。与其他报道的抗肿瘤肽相比,A9K具有更好的杀菌和抗肿瘤活性,对正常细胞具有较低的毒性。现有的大多数自组装短肽只有在特定条件下才能形成具有特定结构的聚集体,并且它们的组装时间相对较长,虽然具有良好的生物相容性,但没有免疫原性。为了进一步优化自组装短肽,LUO等[73]设计具有β-折叠结构的自组装短肽DRF3,具有2个RLDIKVEF结构,有效增加肽链的长度和结构的稳定性,在体外可持续、缓慢地释放抗原,募集并促进树突状细胞成熟;此外,自组装短肽DRF3可以有效抑制肿瘤细胞的增殖和分化,抑制结肠癌细胞的生长。前列腺癌患者前列腺酸性磷酸酶水平升高,基于此,FENG等[74]设计一种自组装短肽分子1-EP能够响应前列腺酸性磷酸酶,前列腺酸性磷酸酶将短肽1-EP去磷酸化为1-E,后者自组装形成均匀的纳米纤维,以剂量依赖性抑制前列腺癌细胞系LNCaP和VCaP生长( IC50分别为 58 μmol/L和 55 μmol/L),为自组装短肽水凝胶治疗前列腺肿瘤提供一种新策略。"

| [1] BRAY F, LAVERSANNE M, SUNG H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. [2] SERENO M, HERNANDEZ DE CÓRDOBA I, GUTIÉRREZ-GUTIÉRREZ G, et al. Brain metastases and lung cancer: molecular biology, natural history, prediction of response and efficacy of immunotherapy. Front Immunol. 2023;14:1297988. [3] CHRISTYANI G, CARSWELL M, QIN S, et al. An Overview of Advances in Rare Cancer Diagnosis and Treatment. Int J Mol Sci. 2024;25(2):1201. [4] CORDANI M, DANDO I, AMBROSINI G, et al. Signaling, cancer cell plasticity, and intratumor heterogeneity. Cell Commun Signal. 2024; 22(1):255. [5] HASAN A, KHAN NA, UDDIN S, et al. Deregulated transcription factors in the emerging cancer hallmarks. Semin Cancer Biol. 2024;98:31-50. [6] MOREDDU R. Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and translational medicine. ADV SCI (WEINH). 2024;11(1):E2304110. [7] PÉREZ-HERRERO E, LANIER OL, KRISHNAN N, et al. Drug delivery methods for cancer immunotherapy. Drug Deliv Transl Res. 2024;14(1): 30-61. [8] POPPER H. Pathologic diagnosis of lung cancer - recent developments. Curr Opin Oncol. 2024;36(1):57-62. [9] SUN J, LUO J, JIANG F, et al. Exploring the cross-cancer effect of circulating proteins and discovering potential intervention targets for 13 site-specific cancers. J Natl Cancer Inst. 2024;116(4):565-573. [10] XU Y, YU Y, YAN R, et al. Modulating β-catenin homeostasis for cancer therapy. Trends Cancer. 2024;10(6):507-518. [11] YAYAN J, FRANKE KJ, BERGER M, et al. Adhesion, metastasis, and inhibition of cancer cells: a comprehensive review. Mol Biol Rep. 2024;51(1):165. [12] YAO Q, LIN F, LU C, et al. A Dual-Mechanism Targeted Bioorthogonal Prodrug Therapy. Bioconjug Chem. 2023;34(12):2255-2262. [13] FALANGA A, BELLAVITA R, BRACCIA S, et al. Hydrophobicity: The door to drug delivery. J Pept Sci. 2024;30(5):e3558. [14] HAN R, HE H, LU Y, et al. Oral targeted drug delivery to post-gastrointestinal sites. J Control Release. 2024;370:256-276. [15] LI S, YU Q, LI H, et al. Self-Assembled Peptide Hydrogels in Regenerative Medicine. Gels. 2023;9(8):653. [16] 卫巍,刘燕飞,何洋,等.β折叠型自组装短肽水凝胶特性及在神经组织工程中的应用前景[J].中国组织工程研究,2018,22(10):1586-1592. [17] HEREMANS J, MAXIMILIAN AWAD R, BRIDOUX J, et al. Sustained release of a human PD-L1 single-domain antibody using peptide-based hydrogels. Eur J Pharm Biopharm. 2024;196:114183. [18] CHAVDA VP, TELI D, BALAR PC, et al. Self-assembled peptide hydrogels for the treatment of diabetes and associated complications. Colloids Surf B Biointerfaces. 2024;235:113761. [19] CURVINO EJ, WOODRUFF ME, ROE EF, et al. Supramolecular Peptide Self-Assemblies Facilitate Oral Immunization. ACS Biomater Sci Eng. 2024;10(5):3041-3056. [20] DAI J, ASHRAFIZADEH M, AREF AR, et al. Peptide-functionalized, -assembled and -loaded nanoparticles in cancer therapy. Drug Discov Today. 2024;29(7):103981. [21] HUA Y,SHEN Y. Applications of self-assembled peptide hydrogels in anti-tumor therapy. Nanoscale Adv. 2024;6(12):2993-3008. [22] KHAZAEI S, VARELA-CALVIÑO R, RAD-MALEKSHAHI M, et al. Self-assembled peptide/polymer hybrid nanoplatform for cancer immunostimulating therapies. Drug Deliv Transl Res. 2024;14(2):455-473. [23] MU R, ZHU D, ABDULMALIK S, et al. Stimuli-responsive peptide assemblies: Design, self-assembly, modulation, and biomedical applications. Bioact Mater. 2024;35:181-207. [24] 卫巍,刘燕飞,张玲,等.自组装短肽水凝胶:止血效应与机制研究进展[J].中国组织工程研究,2019,23(2):310-316. [25] CHEN H, LIU Z, LI L, et al. Peptide Supramolecular Self-Assembly: Regulatory Mechanism, Functional Properties, and Its Application in Foods. J Agric Food Chem. 2024;72(11): 5526-5541. [26] DU Z, FAN B, DAI Q, et al. Supramolecular peptide nanostructures: Self-assembly and biomedical applications. Giant. 2022;9:100082. [27] NAJAFI H, FARAHAVAR G, JAFARI M, et al. Harnessing the Potential of Self-Assembled Peptide Hydrogels for Neural Regeneration and Tissue Engineering. Macromol Biosci. 2024;24(6):e2300534. [28] WILLIAMS-NOONAN BJ, KULKARNI K, TODOROVA N, et al. Atomic Scale Structure of Self-Assembled Lipidated Peptide Nanomaterials. Adv Mater. 2024;36(24):e2311103. [29] LAMPEL A, ULIJN RV, TUTTLE T. Guiding principles for peptide nanotechnology through directed discovery. Chem Soc Rev. 2018; 47(10):3737-3758. [30] KUANG Y, LI Z, CHEN H, et al. Advances in self-assembled nanotechnology in tumor therapy. Colloids and surfaces. Colloids Surf B Biointerfaces. 2024;237:113838. [31] TAO K, WANG J, ZHOU P, et al. Self-assembly of short aβ(16-22) peptides: effect of terminal capping and the role of electrostatic interaction. Langmuir. 2011;27(6):2723-2730. [32] CUI H, MURAOKA T, CHEETHAM AG, et al. Self-assembly of giant peptide nanobelts. Nano Lett. 2009;9(3):945-951. [33] CAPLAN MR, MOORE PN, ZHANG S, et al. Self-assembly of a beta-sheet protein governed by relief of electrostatic repulsion relative to van der Waals attraction. Biomacromolecules. 2000;1(4):627-631. [34] ROBERTS D, ROCHAS C, SAIANI A, et al. Effect of peptide and guest charge on the structural, mechanical and release properties of β-sheet forming peptides. Langmuir. 2012;28(46):16196-16206. [35] TANG C, MILLER AF, SAIANI A. Peptide hydrogels as mucoadhesives for local drug delivery. Int J Pharm. 2014;465(1-2):427-435. [36] GUO Q, LIU Y, MU G, et al. A peptide-drug hydrogel to enhance the anti-cancer activity of chlorambucil. Biomater Sci. 2020;8(20): 5638-5646. [37] CHEN L, ZHAO X, LIU X, et al. Development of small molecule drugs targeting immune checkpoints. Cancer Biol Med. 2024;21(5): 382-399. [38] ZHOU YT, CHENG K, LIU B, et al. Recent progress of nano-drugs in neutron capture therapy. Theranostics. 2024;14(8):3193-3212. [39] CHEN Z, LIU M, WANG N, et al. Unleashing the Potential of Camptothecin: Exploring Innovative Strategies for Structural Modification and Therapeutic Advancements. J Med Chem. 2024;67(5): 3244-3273. [40] FARHOUDI L, MARYAM HOSSEINIKHAH S, VAHDAT-LASEMI F, et al. Polymeric micelles paving the Way: Recent breakthroughs in camptothecin delivery for enhanced chemotherapy. Int J Pharm. 2024;659:124292. [41] CHENG DB, WANG D, GAO YJ, et al. Autocatalytic Morphology Transformation Platform for Targeted Drug Accumulation. J Am Chem Soc. 2019;141(10):4406-4411. [42] LIU J, LIU J, CHU L, et al. Self-assembling peptide of D-amino acids boosts selectivity and antitumor efficacy of 10-hydroxycamptothecin. ACS Appl Mater Interfaces. 2014;6(8): 5558-5565. [43] LIANG W, FAN Y, LIU Y, et al. ROS/pH dual-sensitive emodin-chlorambucil co-loaded micelles enhance anti-tumor effect through combining oxidative damage and chemotherapy. Int J Pharm. 2023; 647:123537. [44] LIANG C, ZHENG D, SHI F, et al. Enzyme-assisted peptide folding, assembly and anti-cancer properties. Nanoscale. 2017;9(33):11987-11993. [45] LI X, CHEN W, REN J, et al. Effects of curcumin on non-alcoholic fatty liver disease: A scientific metrogy study. Phytomedicine. 2024; 123:155241. [46] CHEN G, LI J, CAI Y, et al. A Glycyrrhetinic Acid-Modified Curcumin Supramolecular Hydrogel for liver tumor targeting therapy. Sci Rep. 2017;7:44210. [47] KARAVASILI C, ANDREADIS DA, KATSAMENIS OL, et al. Synergistic Antitumor Potency of a Self-Assembling Peptide Hydrogel for the Local Co-delivery of Doxorubicin and Curcumin in the Treatment of Head and Neck Cancer. Mol Pharm. 2019;16(6):2326-2341. [48] ASHWANIKUMAR N, KUMAR NA, SANEESH BABU PS, et al. Self-assembling peptide nanofibers containing phenylalanine for the controlled release of 5-fluorouracil. Int J Nanomedicine. 2016;11: 5583-5594. [49] WU C, JIAO Q, WANG C, et al. Nanofibrillar peptide hydrogels for self-delivery of lonidamine and synergistic photodynamic therapy. Acta Biomater. 2023;155:139-153. [50] CAO M, LU S, WANG N, et al. Enzyme-Triggered Morphological Transition of Peptide Nanostructures for Tumor-Targeted Drug Delivery and Enhanced Cancer Therapy. ACS Appl Mater Interfaces. 2019;11(18):16357-16366. [51] BAO W,LI Z. Efficacy and safety of neoadjuvant chemotherapy containing anti-angiogenic drugs, immunotherapy, or PARP inhibitors for ovarian cancer. Crit Rev Oncol Hematol. 2024;194:104238. [52] CHEN Z, HU T, ZHOU J, et al. Overview of tumor immunotherapy based on approved drugs. Life Sci. 2024;340:122419. [53] RAUF A, JOSHI PB, OLATUNDE A, et al. Comprehensive review of the repositioning of non-oncologic drugs for cancer immunotherapy. Med Oncol. 2024;41(5):122. [54] DAI X, MENG J, DENG S, et al. Targeting CAMKII to reprogram tumor-associated macrophages and inhibit tumor cells for cancer immunotherapy with an injectable hybrid peptide hydrogel. Theranostics. 2020;10(7):3049-3063. [55] ZHU L, LIU J, QIU M, et al. Bacteria-mediated metformin-loaded peptide hydrogel reprograms the tumor immune microenvironment in glioblastoma. Biomaterials. 2022;288:121711. [56] YANG K, ZHOU Y, HUANG B, et al. Sustained release of tumor cell lysate and CpG from an injectable, cytotoxic hydrogel for melanoma immunotherapy. Nanoscale Adv. 2023;5(7):2071-2084. [57] LI J, SHI K, SABET ZF, et al. New power of self-assembling carbonic anhydrase inhibitor: Short peptide-constructed nanofibers inspire hypoxic cancer therapy. Sci Adv. 2019;5(9):eaax0937. [58] WANG B, CHEN J, CASERTO JS, et al. An in situ hydrogel-mediated chemo-immunometabolic cancer therapy. Nat Commun. 2022; 13(1):3821. [59] BECK JD, DIKEN M, SUCHAN M, et al. Long-lasting mRNA-encoded interleukin-2 restores CD8(+) T cell neoantigen immunity in MHC class I-deficient cancers. Cancer Cell. 2024;42(4): 568-582.e511. [60] WANG J, LU Q, CHEN X, et al. Targeting MHC-I inhibitory pathways for cancer immunotherapy. Trends Immunol. 2024;45(3):177-187. [61] REINIS M, STEPANEK I, SIMOVA J, et al. Induction of protective immunity against MHC class I-deficient, HPV16-associated tumours with peptide and dendritic cell-based vaccines. Int J Oncol. 2010; 36(3):545-551. [62] HAO W, HU C, HUANG Y, et al. Coadministration of kla peptide with HPRP-A1 to enhance anticancer activity. PLoS One. 2019;14(11): e0223738. [63] STANDLEY SM, TOFT DJ, CHENG H, et al. Induction of cancer cell death by self-assembling nanostructures incorporating a cytotoxic peptide. Cancer Res. 2010;70(8):3020-3026. [64] LIU T, LI P, JIN H, et al. Influence of designer self-assembling nanofiber scaffolds containing anti-cancer peptide motif on hepatoma carcinoma cells. J Biomed Mater Res A. 2017;105(8):2329-2334. [65] KUANG Y, DU X, ZHOU J, et al. Supramolecular nanofibrils inhibit cancer progression in vitro and in vivo. Adv Healthc Mater. 2014;3(8): 1217-1221. [66] FENG Z, HAN X, WANG H, et al. Enzyme-Instructed Peptide Assemblies Selectively Inhibit Bone Tumors. Chem. 2019;5(9):2442-2449. [67] CASTELLETTO V, EDWARDS-GAYLE CJC, GRECO F, et al. Self-Assembly, Tunable Hydrogel Properties, and Selective Anti-Cancer Activity of a Carnosine-Derived Lipidated Peptide. ACS Appl Mater Interfaces. 2019;11(37):33573-33580. [68] JEENA MT, PALANIKUMAR L, GO EM, et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat Commun. 2017;8(1):26. [69] WANG H, FENG Z, WANG Y, et al. Integrating Enzymatic Self-Assembly and Mitochondria Targeting for Selectively Killing Cancer Cells without Acquired Drug Resistance. J Am Chem Soc. 2016;138(49):16046-16055. [70] GHOSH T, WANG S, KASHYAP D, et al. Self-assembled benzoselenadiazole-capped tripeptide hydrogels with inherent in vitro anti-cancer and anti-inflammatory activity. Chem Commun (Camb). 2022;58(54):7534-7537. [71] LUO S, FENG J, XIAO L, et al. Targeting self-assembly peptide for inhibiting breast tumor progression and metastasis. Biomaterials. 2020;249:120055. [72] XU H, CHEN C X, HU J, et al. Dual modes of antitumor action of an amphiphilic peptide A(9)K. Biomaterials. 2013;34(11):2731-2737. [73] LUO R, WAN Y, LUO X, et al. A Rapid Self-Assembly Peptide Hydrogel for Recruitment and Activation of Immune Cells. Molecules. 2022; 27(2):419. [74] FENG Z, WANG H, YI M, et al. Instructed-Assembly of Small Peptides Inhibits Drug-Resistant Prostate Cancer Cells. Pept Sci (Hoboken). 2020;112(1):10. [75] ABDEL-RAHMAN RM, ABDEL-MOHSEN AM, FRANKOVA J, et al. Self-Assembled Hydrogel Membranes with Structurally Tunable Mechanical and Biological Properties. Biomacromolecules. 2024;25(6):3449-3463. [76] HATAMI H, RAHIMAN N,MOHAMMADI M. Oligonucleotide based nanogels for cancer therapeutics. Int J Biol Macromol. 2024;267(Pt 2): 131401. [77] HIRTH E, CAO W, PELTONEN M, et al. Self-assembled and perfusable microvasculature-on-chip for modeling leukocyte trafficking. Lab Chip. 2024;24(2):292-304. [78] LIU G, MA R, LIU P, et al. An injectable nanocomposite hydrogel prevents postoperative tumor recurrence and wound infection via synergistic photothermal-chemo-therapy. J Colloid Interface Sci. 2024;655:809-821. [79] LIU Y, OKESOLA BO, OSUNA DE LA PEÑA D, et al. A Self-Assembled 3D Model Demonstrates How Stiffness Educates Tumor Cell Phenotypes and Therapy Resistance in Pancreatic Cancer. Adv Healthc Mater. 2024;13(17):e2301941. [80] ZHU H, WU J, ZHAO J, et al. Dual-functional DNA nanogels for anticancer drug delivery. Acta Biomater. 2024;175:240-249. [81] ZHU W, ZHOU Z, YANG M, et al. Injectable Nanocomposite Immune Hydrogel Dressings: Prevention of Tumor Recurrence and Anti-Infection after Melanoma Resection. Small. 2024; 20(28):e2309476. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [3] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [4] | Li Shuai, Liu Hua, Shang Yonghui, Liu Yicong, Zhao Qihang, Liu Wen. Stress distribution on the maxilla when wearing the Twin-block appliance for Class II malocclusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 881-887. |
| [5] | Ding Zhili, Huang Jie, Jiang Qiang, Li Tusheng, Liu Jiang, Ding Yu. Constructing rabbit intervertebral disc degeneration models by different methods under X-ray guidance: a comparative study [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 995-1002. |
| [6] | Zhao Hongxia, Sun Zhengwei, Han Yang, Wu Xuechao , Han Jing. Osteogenic properties of platelet-rich fibrin combined with gelatin methacryloyl hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 809-817. |
| [7] | Xiao Fang, Huang Lei, Wang Lin. Magnetic nanomaterials and magnetic field effects accelerate bone injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 827-838. |
| [8] | Wang Zilin, Mu Qiuju, Liu Hongjie, Shen Yuxue, Zhu Lili. Protective effects of platelet-rich plasma hydrogel on oxidative damage in L929 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 771-779. |
| [9] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [10] | Li Mingzhe, Ye Xiangling, Wang Bing, Yu Xiang. Preparation and osteogenic properties of liquid crystal display light-cured polylactic acid scaffold loaded with nano-tantalum [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 670-677. |
| [11] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [12] | Dong Meilin, Du Haiyu, Liu Yuan. Quercetin-loaded carboxymethyl chitosan hydrogel promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 692-699. |
| [13] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [14] |
Zhang Bo, Zhang Zhen, Jiang Dong.
Tannic acid modified interpenetrating network hydrogel promotes tissue remodeling of ruptured Achilles tendon after surgery#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 721-729.
|
| [15] | Dang Xiaowen, Huang Hailiang, Huang Lei, Wang Yajie . Research frontiers and hotspots of carbon nanomaterials in biomedical field over the past 10 years [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 752-760. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||