Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (18): 3856-3867.doi: 10.12307/2025.667
Previous Articles Next Articles
Molecular mechanism of active ingredients of Ligustri Lucidi Fructus against osteoporosis
Wang Wenchi1, 2, Xia Tian2, Wu Ruiqi1, 2, Liang Haohan3, Ni Zhenyang1, 2, Zhang Zhenhao1, 2, Li Zhenxing4, Chen Guanghui4, Su Han4
- 1Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 2Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 3Taihe Hospital of Wannan Medical College, Fuyang 236600, Anhui Province, China; 4First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China
-
Received:2024-07-16Accepted:2024-08-28Online:2025-06-28Published:2024-11-28 -
Contact:Li Zhenxing, Associate chief physician, Associate professor, Master’s supervisor, First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Wang Wenchi, Master candidate, Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
Supported by:Guangxi Medical and Healthcare Appropriate Technology Development and Popularization and Application Project, No. S2022053; Guangxi Universities and Colleges Young and Middle-aged Teachers’ Scientific Research Basic Ability Enhancement Project, No. 2021KY0302; Guangxi High-level Chinese Medicine Key Discipline Construction Project, No. 2024016-02-04; Guangxi Traditional Chinese Medicine Key Research Laboratory Construction Project - Guangxi Traditional Chinese Medicine Meridian and Tendon Rehabilitation Key Research Laboratory, No. 2024019-03; Guangxi Key Cultivation Discipline Construction Project of Traditional Chinese Medicine, No. GZXK-Z-20-32; Guangxi University of Traditional Chinese Medicine High-level Talent Team Construction Project - High-level Talent Cultivation Innovative Team, No. 2022A006; Guangxi Seventh Batch of National Old Chinese Medicine Experts Academic Experience Inheritance Work Project in 2024 (to XT); 2024 Guangxi Chinese Medicine Appropriate Technology Development and Promotion Project, No. GZSY22-39 (to XT); 2023 Guangxi University of Chinese Medicine Doctoral Research Initiation Fund Project, No. 2023BS018 (to XT)
CLC Number:
Cite this article
Wang Wenchi, Xia Tian, Wu Ruiqi, Liang Haohan, Ni Zhenyang, Zhang Zhenhao, Li Zhenxing, Chen Guanghui, Su Han. Molecular mechanism of active ingredients of Ligustri Lucidi Fructus against osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3856-3867.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
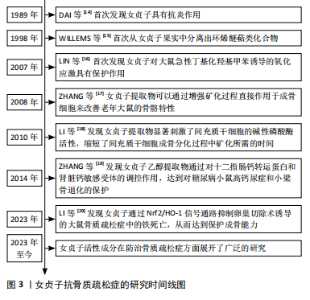
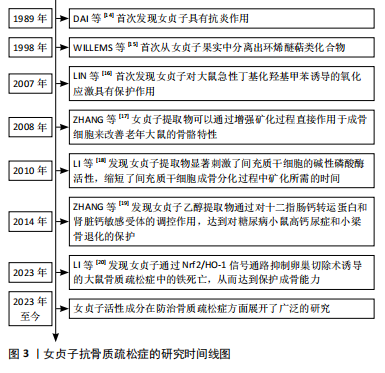
2.1 女贞子抗骨质疏松的研究时间图 自1989年DAI等[14]开创性地揭示女贞子具备抗炎功效以来,科学家们对女贞子的研究不断深入,逐步解锁了其丰富的药理价值。在1998年,WILLEMS等[15]从女贞子果实中成功分离出环烯醚萜类化合物,为后续的深入研究奠定了物质基础。进入21世纪,女贞子在防治氧化应激及骨骼健康领域的潜力逐渐显现。在2007年,LIN等[16]的研究首次展示了女贞子对大鼠急性氧化应激模型的显著保护作用,揭示了其在抗氧化领域的潜力。在2008年,ZHANG等[17]的研究则进一步证明了女贞子提取物能直接作用于成骨细胞,通过增强矿化过程来改善老年大鼠的骨骼特性,为骨质疏松症的治疗提供了新的思路。随着研究的深入,女贞子在促进骨骼再生方面的作用也日益受到重视。在2010年,LI等[18]的研究表明,女贞子提取物能显著激活间充质干细胞的碱性磷酸酶活性,加速其成骨分化过程,为骨骼修复和再生提供了有力的支持。而到了2014年,ZHANG等[19]的研究又揭示了女贞子乙醇提取物在糖尿病相关骨骼疾病中的保护作用,通过调控钙转运蛋白和钙敏感受体,有效对抗糖尿病小鼠的高钙尿症和小梁骨退化,进一步拓宽了女贞子在骨骼健康领域的应用范围。直至最近的2023年,LI等[20]的研究更是创新性地提出了女贞子通过Nrf2/血红素加氧酶1(heme oxygenase,HO-1)信号通路抑制卵巢切除诱导的大鼠骨质疏松症中的铁死亡,从而保护成骨能力的新机制,为女贞子在骨质疏松症防治中的应用提供了更为深入的科学依据。研究时间线介绍见图3。"
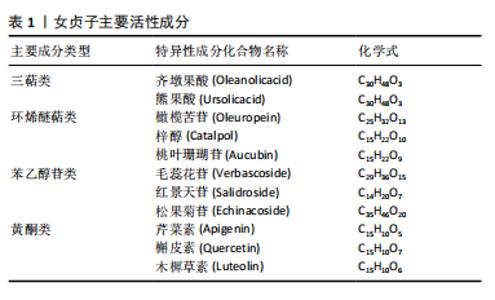
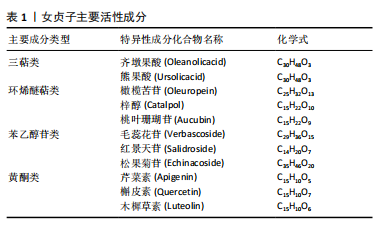
2.2 女贞子活性成分治疗骨质疏松症 根据现代药物学的研究,从女贞子中提取的活性物质主要涵盖了三萜类、环烯醚萜类、苯乙醇苷以及黄酮类这4种主要化合物,见表1。女贞子的治疗主要依赖于这些成分,这些成分具有多种药理效应,包括抗骨质疏松症、抗氧化、抗炎、抗肿瘤以及降低血脂等。此外还含有挥发油、多糖和脂肪酸等多种活性成分,经相关研究报道,这些活性成分在抗骨质疏松症方面也展现出了独特的潜力和广阔的开发前景,但是目前相关研究相对较少,为此在未来的研究当中去挖掘相应的活性成分尤为重要。在临床应用方面,以女贞子为主药组方制成复方制剂或单味中药注射剂已广泛应用于各种疾病的辅助治疗及预防。最近几年的研究揭示,女贞子中含有的一些活性成分具有调控相关信号通路的方式促进相关蛋白的表达,因此已经成为当下防治骨质疏松症药物研究领域中的关注焦点。"
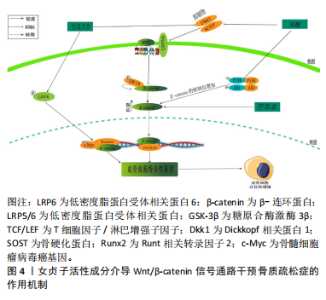
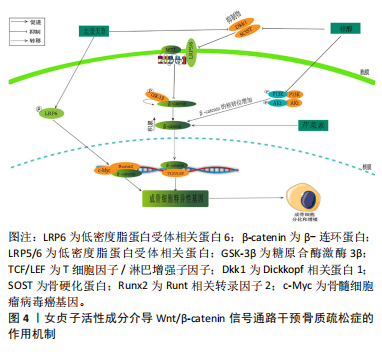
2.2.1 三萜类化合物 三萜类化合物属于萜类家族,它们广泛存在于植物、真菌和某些动物当中,存在形式多样,包括游离状态、糖苷形式以及作为植物甾醇和植物激素的成分,在龙胆科等植物中较为常见[21],萜类化合物一直是天然药物化学成分研究的活跃领域。研究揭示,三萜类化合物在治疗骨质疏松症的过程中,其多种生物活性起到了至关重要的作用。齐墩果酸、熊果酸女贞子提取物,在防治骨质疏松症方面具有良好的天然优势。YU等[22]研究发现,在双侧卵巢切除模型中,齐墩果酸显著增加小鼠骨骼CYP27B1(25-羟基维生素D31-α-羟化酶)表达和循环25-羟基维生素[1,25(OH)2D3];此外,又上调了人成骨样MG-63细胞中CYP27B1蛋白的表达和活性,以及维生素D应答骨标志物碱性磷酸酶活性和骨桥蛋白的表达,从而发挥治疗骨质疏松症的作用。 另一方面,YANG等[23]在强的松龙诱导的斑马鱼模型中,当齐墩果酸浓度为1 μmol/L时,斑马鱼的矿化面积显著增加,累积光密度显著增加,从而展现出显著的抗骨质疏松作用。与此同时,XU等[24]研究发现,在糖皮质激素诱导的SD雌性成年大鼠模型中,齐墩果酸能显著逆转糖皮质激素引起的骨密度下降,同时齐墩果酸能显著升高血钙,从而增强体内破骨细胞的功能,导致血钙水平升高,减少骨质流失,并抑制破骨细胞的生长。另外,CAO等[25]在卵巢切除后的C57BL/6J小鼠模型中,发现齐墩果酸能显著抑制尿钙排泄,增加骨密度,并且在另一方面增加1,25(OH)2D3的水平。ZHENG等[26]研究表明,在双侧卵巢切除术的雌性大鼠中,证实了熊果酸通过改善骨小梁的微结构恶化,减少椎骨中抗酒石酸酸性磷酸酶阳性破骨细胞的数量以及降低血清中破骨细胞特异性细胞因子的释放,其次在体外实验中发现,熊果酸明显降低了巨噬细胞中破骨特殊标记蛋白(proto-oncogene protein,c-Fos)和NFATc1的表达。并且,CHENG等[27]在维甲酸诱导的SD雌性大鼠模型中发现,熊果酸可防止骨质流失和小梁微结构的恶化,从而维持骨骼的生物力学能力,并且升高碱性磷酸酶和骨钙素的含量。这表明熊果酸能够增加成骨细胞的活性,降低破骨细胞的活性,从而发挥抗骨质疏松症的作用。 2.2.2 环烯醚萜类化合物 环烯醚萜苷类化合物(iridoid/secoiridoidglycosides)属于女贞子的一种主要活性成分,常见于龙胆科植物当中[28]。研究表明,环烯醚萜苷类化合物在治疗骨质疏松症方面表现出了优越的成效。橄榄苦苷、梓醇及桃叶珊瑚苷是从中药女贞子中分离出得到的,在治疗骨质疏松症及抗氧化等方面展现出了优异的疗效。LAO等[29]研究表明,在高糖诱导的小鼠骨髓间充质干细胞模型中,证明了橄榄苦苷在高糖微环境下可以促进骨髓间充质干细胞增殖和分化。并且在另一方面也证实橄榄苦苷能上调翼状螺旋相关转录因子10b(Wnt10b)以及成骨相关基因中的成骨细胞特异性转录因子2(runt-related transcriptionfactor 2,Runx2)、骨成形特异性转录因子(Osterix)和骨钙素,从而发挥抗骨质疏松症的作用。SANTIAGO- MORA等[30]在以人骨髓间充质干细胞为模型的研究中,证实了橄榄苦苷能够促进成骨细胞分化增加,并且降低脂肪细胞的分化,并且增加了成骨细胞生成标志物Runx2、Osterix、Ⅰ型胶原、骨钙素或碱性磷酸酶的基因表达。CHEN等[31]在双侧卵巢切除建立骨质疏松症大鼠模型中,发现梓醇可以通过激活信号转导与转录激活因子3(signaltransducersandactivatorsoftranscription3,STAT3)从而提高骨髓间充质干细胞的成骨分化,并抑制其向脂肪细胞的分化,并且促进新生血管壁的生成。CHENG等[32]在高糖诱导MC3T3细胞模型中,发现高糖环境会导致MC3T3-E1细胞的增殖和矿化能力下降,同时降低碱性磷酸酶活性;经一定浓度的梓醇处理后,碱性磷酸酶活性得到提高,进一步证实高糖环境诱导下的成骨调节是通过Wnt/β-catenin途径发生的。 2.2.3 苯乙醇苷类化合物 苯乙醇苷类化合物 (phenylethanoid glycosides)主要由β-葡萄糖构成,是一种在双子叶植物中普遍存在的天然糖苷。特别是在女贞子中,已分离出多个此类化合物,以毛蕊花糖苷、红景天苷和松果菊苷等为主。LI等[33]研究表明,在双侧卵巢切除建立的骨质疏松症模型小鼠中,毛蕊花糖苷主要是通过下调RANKL/RANK通路、抑制核转录因子κB从而调节下游蛋白靶点的活性,增加骨小梁的数量。与此同时MARTINAKOVA等[34]发现在双侧切除卵巢建立骨质疏松症小鼠模型中,毛蕊花糖苷具有显著降低肿瘤坏死因子α和白细胞介素6导致的炎症反应的能力,从而影响成骨细胞的生长和分化。CHEN等[35]在双侧卵巢切除小鼠和糖尿病小鼠的模型中,用红景天苷(20 mg/kg)治疗6周后发现,红景天苷增加了骨钙水平,减少尿钙排泄,从而减轻双侧卵巢切除小鼠和糖尿病小鼠骨小梁的恶化,其作用机制是通过调节维生素D代谢和跨细胞钙转运蛋白以及调节肾脏中钙敏感受体(calcium-sensing receptor,CaSR)的表达来改善钙稳态,从而发挥骨保护作用。XIE等[36]在经地塞米松诱导的斑马鱼模型中,发现红景天苷显著缓解了骨形成的抑制作用,促进了斑马鱼颅骨的矿化,其作用机制是通过TGF-β/Smad2/3通路改善经地塞米松诱导的对成骨分化和骨形成的抑制。LI等[37]在去卵巢骨质疏松大鼠模型中,发现了松果菊苷通过促进骨形成和抑制骨吸收而具有保护作用;此外,免疫组化染色证实显著增强了子宫内雌激素受体(estrogen receptor,ER)的表达,对预防雌激素缺乏所致的骨质疏松症具有良好的优势。 2.2.4 黄酮类化合物 黄酮类化合物(Flavonoids)来源于植物中的多酚类物质,具有2-苯基色原酮(C6-C3-C6)的基本结构,并展现出多种生物活性。目前,已从女贞子中分离出多种黄酮类化合物,主要包括芹菜素、槲皮素和木樨草素等,这些化合物表现出促进成骨和抑制破骨等多种药理活性。在对抗骨质疏松症方面发挥着至关重要的作用。ALI等[38]在双侧卵巢切除建立的骨质疏松症小鼠模型中,证实芹菜素能显著抑制双侧卵巢切除小鼠股骨小梁骨丢失,并且在体外实验中发现芹菜素可以抑制多核破骨细胞的形成,从而达到防治骨质流失的作用。CHOI等[39]在成骨细胞MC3T3-E1细胞模型中,用芹菜素(10 μmol/L)处理过后,细胞中碱性磷酸酶活性和胶原含量显著升高,并且在另一方面减少了成骨细胞中肿瘤坏死因子α诱导的白细胞介素6和一氧化氮的产生,从而达到防治骨质疏松的作用。此外SUN等[40]研究发现,在睾丸切除术后的C57BL/6小鼠模型中,经槲皮素治疗后胰岛素样生长因子1和高密度脂蛋白水平升高,参与葡萄糖摄取的蛋白质的表达增加,而参与脂质产生的蛋白质的表达降低;此外,GPRC6A和磷酸化腺苷单磷酸活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)/AMPK在肝脏和胫骨组织中的表达比值升高。相比之下,槲皮素组的磷酸化哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)/mTOR比值降低。从而可以说明槲皮素可以减少睾酮缺乏引起的骨质疏松症,其有益作用可能与通过GPCR6A/AMPK/mTOR信号通路调节葡萄糖代谢和抑制脂质代谢有关。ZHENG等[41]在经糖皮质激素诱导SD大鼠颅骨成骨细胞中,发现木犀草素促进成骨细胞增殖、抑制成骨细胞凋亡和后期分化,这种功能可能与上调Sema3A/NRP1/PlexinA1的表达有关。KIM等[42]发现,在双侧卵巢切除构建的骨质疏松症大鼠模型中,口服木犀草素[5,20 mg/(kg·d)]后可显著提高双侧卵巢切除小鼠股骨小梁和皮质骨的骨密度和骨矿物质含量,防止双侧卵巢切除手术引起的骨强度指标下降。血清生化指标分析也显示木犀草素可阻止双侧卵巢切除诱导的骨转换增加。 2.2.5 其他化学成分 目前从女贞子中还分离出了如挥发油、多糖和脂肪酸等多样的化学成分。女贞子的挥发油包括大根香叶烯D、7-杜松醇和石竹烯等成分。YAMAGUCHI等[43]在体外对小鼠骨髓细胞的培养实验中发现,β-石竹烯能够刺激成骨细胞的矿化过程,同时抑制脂肪和破骨细胞的生成,从而有效预防和治疗骨质疏松症的出现。同时,SHAN等[44]在MC3T3-E1成骨细胞模型研究中发现,经过反式石竹烯处理后,胶原蛋白的含量、碱性磷酸酶的活性、骨钙素的生成和矿化都有了显著的提升;此外,在抗霉素A暴露之前使用反式石竹烯进行预处理,可以有效地减少细胞死亡、防止活性氧的释放以及阻止成骨细胞功能的障碍,从而显著降低抗霉素A引发的细胞损害。然而女贞子多糖的成分包括蔗糖、鼠李糖、阿拉伯糖、葡萄糖和岩藻糖等,而其中的脂肪酸主要是不饱和脂肪酸,如棕榈酸、棕榈油酸和硬脂酸等。目前,关于女贞子中分离的多糖和脂肪酸等化学物质在对抗骨质疏松症方面的研究报道相对有限。为了更深入地了解多糖和脂肪酸在骨质疏松预防和治疗中的分子作用机制,需要进一步研究它们与骨细胞的交互过程以及信号传导路径的调节方式,为后期相应药物的开发提供依据与参考。 2.3 女贞子活性成分治疗骨质疏松症的相关信号通路 2.3.1 Wnt/β-catenin通路 Wnt/β-catenin通路在骨骼分化过程中扮演了关键角色,可以激发一系列与骨骼代谢和成长有关的核心调节因子的产生,Wnt介导的Wnt-β-catenin信号转导是通过与frizzed(Fz)家族和LRP5/6的七通跨膜受体相互作用而激活的。当wnt与Fz受体和LRP5/6的稳定且富含半胱氨酸的部位结合时,无规则蛋白(dishevelled,Dvl)的细胞质蛋白会受到刺激。Dvl具有阻断轴抑制蛋白(axis Inhibitor,Axin)的能力,它可以阻止由大肠腺瘤性息肉病、轴抑制蛋白、糖原合成酶激酶3β和酪蛋白激酶1α构成的破坏性复合物的生成。β-catenin的磷酸化和相应的降解被阻止了,β-catenin是Wnt-β-catenin信号通路中的关键肿瘤细胞增殖调节因子。随后,β连环蛋白稳定易位进入细胞核,并与T细胞因子/淋巴增强子因子(T-cell factor/lymphoid enhancer factor,TCF/LEF)转录复合物结合从而激活下游靶基因的转录,发挥其相应的功能[45-46]。 LIU等[47]在双侧卵巢切除大鼠骨质疏松症模型中,发现梓醇能够抑制骨质疏松症大鼠Dkk1抑制物和骨硬化蛋白(SOST)的表达。并且通过激活TCF/LEF转录因子促进Runx2的活性,从而发挥其成骨作用。此外,ZHU等[48]研究表明,在双侧卵巢切除建立骨质疏松症大鼠模型中,梓醇可促进骨髓间充质干细胞成骨分化并防止骨质流失,经一定浓度的梓醇处理后发现核β-catenin的表达水平显著增强,表明梓醇在促进成骨作用方面与Wnt/β-catenin通路的激活有关。LIU等[49]在双侧卵巢切除大鼠和原代成骨细胞中,发现红景天苷通过抑制SOST和Dkk1的表达来激活Wnt/β-catenin信号通路,从而增加双侧卵巢切除大鼠和原代成骨细胞磷酸化低密度脂蛋白受体相关蛋白6(phosphorylated low-density lipoprotein receptor-related protein 6,p-LRP6)表达,降低糖原合酶激酶3β(glycogen synthase kinase 3 Beta,GSK3β)表达,增加细胞核β-catenin,Runx2和c-Myc的表达,来促进成骨细胞骨形成。PAN 等[50]在人间充质干细胞模型中,发现芹菜素可增强Wnt信号通路关键转录因子β-catenin及多个下游靶基因的表达,从而激活Wnt/β-catenin信号通路。从而达到防治骨质疏松症的作用,见图4。"
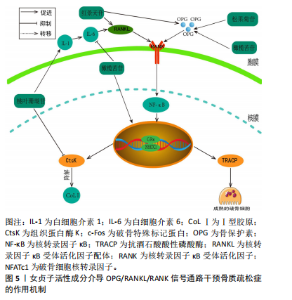
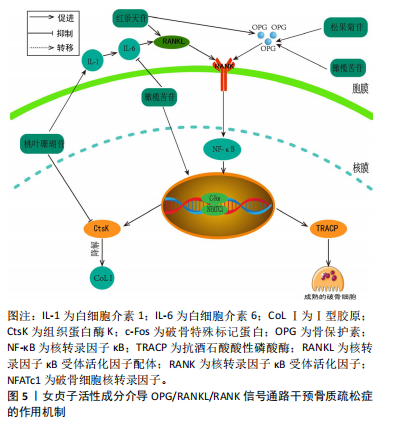
2.3.2 骨保护素/RANKL/RANK通路 骨保护素/RANKL/RANK通路在骨骼重建和破骨细胞分化的过程中,扮演着关键的信号传递角色[51]。RANKL与RANK结合形成复合物会激活下游信号通路,从而激活破骨细胞相关基因的表达[52]。骨保护素是由成骨细胞产生的,作为RANKL的诱饵受体,它是破骨细胞的唯一负向调节因子,能够阻止破骨细胞与RANKL的结合,中断信号的传递,进而避免骨的吸收。白细胞介素1是一种炎性细胞因子,能通过激活白细胞介素6提高RANKL的表达。当转录因子κB受体与RANKL结合时,它会促进破骨细胞的生长。此外,RANKL和扮演着促进c-Fos与NFATc1的结合的角色,促使破骨细胞中的抗酒石酸酸性磷酸酶(Tartrate-Resistant Acid Phosphatase,TRACP)和组织蛋白酶K(Cathepsin K,CtsK)等基因活性在NFATc1的作用下迅速被激活,从而发挥其功能。此外,CtsK还具备分解Ⅰ型胶原的特性,这进一步影响了骨骼的代谢过程,并加速了骨骼的流失[53-54]。 ZHENG等[55]研究发现,在去卵巢切除合并糖尿病的SD大鼠模型中,当红景天苷的浓度为20 mg/kg灌胃处理后,实验结果表明红景天苷能抑制骨转换增加,抑制骨髓脂肪生成,并且上调去卵巢切除糖尿病大鼠骨保护素/RANKL的蛋白和mRNA比值,从而能有效改善糖尿病大鼠骨质量。此外,LI等[56]研究表明,在小鼠颅骨分离MC3T3-E1细胞模型中,松果菊苷在促进成骨细胞分化和矿化方面起到了积极作用,这涉及到Ⅰ型胶原的释放和骨保护素的形成。并且能降低RANKL蛋白的表达,从而促进成骨细胞的增殖与分化并且减少破骨细胞的产生。LIU等[57]在双侧卵巢切除的雌性大鼠中发现,橄榄苦苷能显著提高SD大鼠的骨密度;同时,它能降低终末白细胞介素6、碱性磷酸酶浓度,对Ca2+浓度无影响;并且在体外实验中,橄榄苦苷能促进成骨细胞的增殖,此外,还能促进骨保护素蛋白和mRNA的表达;相反,它抑制RANKL蛋白和mRNA的表达,其作用机制与调节骨保护素/RANKL的表达有关。ZHANG等[58]发现,在注射地塞米松诱导的骨质疏松症小鼠模型中,桃叶珊瑚苷能有效地减少小鼠血清中白细胞介素1的浓度,并进一步降低RANKL细胞中NFATc1和CtsK的表达水平,进而达到减少骨质流失的作用,见图5。"
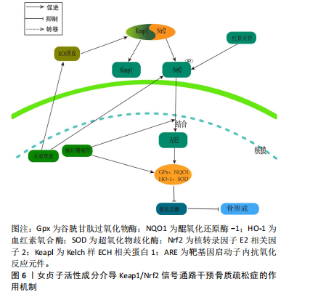
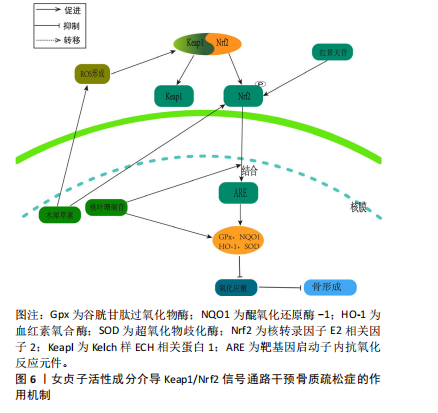
2.3.3 Keap1/Nrf2通路 氧化应激的发生是由于氧化与抗氧化间的平衡遭到破坏,这种不平衡促使破骨细胞分化,进而触发骨质疏松症。细胞氧化还原平衡的关键是对Nrf2的调控[59]。正常生理环境下,在缺少keap1与Neh2结构域的前提下,Nrf2表现为一种不稳定的蛋白质。然而,在稳定的环境中,Keap1是E3泛素连接酶的组成部分,该系统利用特定的转录因子Nrf2来达到泛素化和依赖于蛋白酶体的分解,进而精确地调节Nrf2的功能表现。在应激反应中,Keap1里的传感器半胱氨酸所驱动的复杂分子结构确保了Nrf2可以规避泛素化的风险,在细胞内累积,并被转运到细胞核[60]。当细胞受到大量活性氧刺激后,叉头转录因子O亚型(forkheadbox O,FoxO)会被激活。但是,当Nrf2经过磷酸化作用后,它会与Keap1分离并转移到细胞核中,与并与靶基因启动子内抗氧化反应元件(antioxidantresponse elementt,ARE)发生结合,这种结合导致了抗氧化酶的生成,包括血红素氧合酶(heme oxygenase-1,HO-1)、醌氧化还原酶1(NADH-oxidoreductase-1,NQO1)、谷胱甘肽过氧化物酶(glutathione peroxidase,GPx)以及超氧化物歧化酶,从而抑制了氧化应激的发生[61]。多项研究证实,Nrf2在抗氧化和抗炎作用中,HO-1起到了关键作用[62]。这些内源性的保护性物质由酶降解后的产物构成,具有抗炎和抗氧化的特性[63-64]。这有助于抑制活性氧对细胞的刺激作用,从而进一步减少活性氧对骨细胞生成的抑制作用。 WANG等[65]在叔丁基过氧化氢(t-BHP)诱导的大鼠成骨细胞中,发现红景天苷降低了活性氧和脂质过氧化水平,提高了谷胱甘肽过氧化物酶和超氧化物歧化酶等抗氧化酶的活性,促进了成骨分化;相关机制研究表明,红景天苷是通过上调Nrf2表达并促进其核易位,阻止了骨质疏松症发展,减少了氧化损伤。此外WANG等[66]发现,在人骨髓间充质干细胞细胞模型中,桃叶珊瑚苷具备促进Nrf2从细胞质向细胞核转位的能力,并在体外条件下显著上调了HO-1与NQO1蛋白表达水平,从而减轻氧化压力导致的成骨抑制。这意味着桃叶珊瑚苷对抗氧化压力的能力是通过Nrf2所介导的信号途径来达成的。 SUH等[67]研究表明,在经甲基乙二醛诱导的MC3T3-E1成骨细胞模型中,木樨草素显著抑制甲基乙二醛诱导MC3T3-E1细胞活性氧生成和肿瘤坏死因子α水平升高以及线粒体超氧化物和心磷脂过氧化的产生,此外,木犀草素增加了谷胱甘肽和Nrf2水平,并降低了甲基乙二醛对HO-1活性的抑制,其作用机制是通过Nrf2/HO-1激活达到细胞保护的作用,见图6。 2.3.4 PI3K/Akt信号通路 PI3K/Akt信号通路被认为是RANKL触发的破骨细胞生成的关键[68]。RANKL/RANK的复合物触发了破骨细胞PI3K的激活和Akt的磷酸化过程,这进一步促进了与破骨细胞相关的关键转录因子原癌基因c-Fos的活性,从而介导破骨细胞的分化和成熟[69]。NFATc1是一种具有特异性的转录因子,能够调控破骨细胞特异性基因(Acp5及CtsK等)以及RANKL/RANK复合物介导的破骨细胞的分化和功能激活。Akt在经过磷酸化大量积累后迅速提高c-Fos的活化,从而导致抗酒石酸酸性磷酸酶5(Tartrate-Resistant Acid Phosphatase 5,TRACP5)"
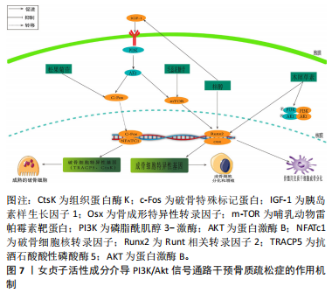
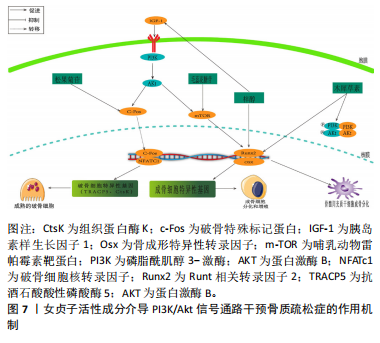
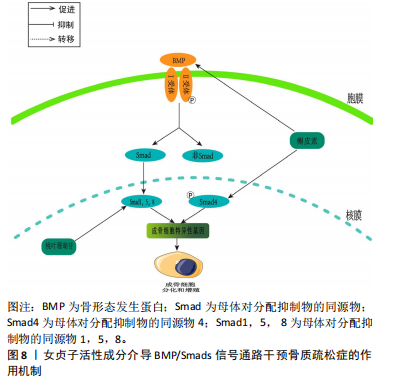
和CtsK的表达水平上升[70]。胰岛素生长因子1在成骨细胞的合成和代谢过程中起到了关键的调节作用[71]。成骨细胞分化中的PI3K/Akt/mTOR信号通路和Runx2相互作用密切。Runx2有助于提高PI3K亚基和Akt的蛋白质表达,影响PI3K/Akt/mTOR通路推动了成骨细胞的增长。 JIANG等[72]在假体周围关节感染大鼠模型中,发现松果菊苷能够通过降低PI3K的表达、减少Akt的磷酸化程度和减少c-Fos的表达,进而有效地抑制破骨细胞中PI3K/Akt/c-Fos的通路,从而实现降低破骨细胞的增殖和成熟能力。LI等[73]研究表明,在注射地塞米松诱导的骨质疏松症小鼠模型中,发现地塞米松能够抑制PI3K/Akt/mTOR通路中的关键蛋白水平,而毛蕊花糖苷则能够减轻这种抑制效果,同时还能提高成骨细胞的活性,从而表明了PI3K/Akt途径中相应的靶点可以被毛蕊花糖苷所识别。GONG等[74]在糖尿病骨质疏松症大鼠模型中,梓醇通过活化PI3K/Akt/mTOR通路促进了胰岛素生长因子1的分泌,并在成骨细胞MC3T3-E1中上调了p-mTOR,p-IGF-1R及p-PI3K的蛋白表达水平。此过程进而激活了对Runx2的调控,强化了成骨细胞的活性,并有效减缓了骨质疏松症的发展进程。LIANG等[75]发现,在双侧卵巢切除构建的骨质疏松症大鼠模型中,木犀草素可减轻双侧卵巢切除大鼠骨质流失,上调骨髓间充质干细胞中Ⅰ型胶原、骨桥蛋白和Runx2蛋白的表达,促进成骨分化。与此同时,体外细胞研究表明,木犀草素通过增加p-PI3K/PI3K和p-Akt/Akt的表达比例,增强了PI3K/Akt信号通路的活性,从而促进骨髓间充质干细胞的成骨分化。见图7。 2.3.5 骨形态发生蛋白/Smads通路 骨形态发生蛋白也被称为骨形成蛋白,它是转化生长因子(transforming growth factor-β,TGF-β)超家族中的一个成员,并在骨基质中有着广泛的分布[76]。骨形态发生蛋白2作为骨形态发生蛋"
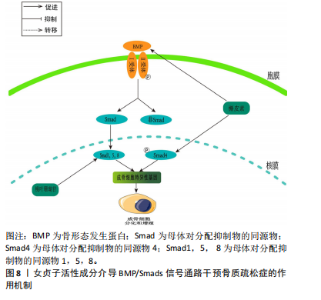
白配体家族的一员,其具有较强的促进骨形成的活性,它能有效地增强骨钙素的活性,从而有助于骨骼和软骨的形成。骨形态发生蛋白与Ⅰ型和Ⅱ型跨膜Ser/Thr激酶受体结合,激活Smad及非Smad信号通路[77]。Smad蛋白作为信号传递者,将骨形态发生蛋白信号由受体转至靶基因,通过磷酸化Smad1,5,8等转导因子并引导其入核,启动Runx2的转录,进而增强早期成骨mRNA的合成[78],此过程调控了骨桥蛋白、碱性磷酸酶及骨钙素等成骨分化标志物的表达,最终促进骨形成。 LI等[79]研究发现,在人成骨样细胞MG63细胞模型中,桃叶珊瑚苷显著提高了Smad1/5/8的表达水平,并增强了碱性磷酸酶和骨钙素的活性,从而进一步加速了骨骼的形成,这也充分证实了骨形态发生蛋白2介导的Smads信号传导在促进骨细胞分化中的关键作用。LI等[80]在注射地塞米松诱导的骨质疏松症小鼠模型中,发现桃叶珊瑚苷通过增加MG63的细胞数量,并在骨质疏松症小鼠模型中通过激活骨形态发生蛋白/Smads信号通路,增强骨形态发生蛋白2和p-Smads的表达,从而促进细胞增殖分化,并展现出对抗骨质疏松症的效果。郑红等[81]研究发现,在双侧卵巢切除建立的骨质疏松症大鼠模型中,经检测发现血液中环磷酰胺、骨形成标志物(PINP)和骨碱性磷酸酶的含量显著上升,而Ca、P和骨密度显著下降。经过槲皮素灌胃处理后,这些现象都有所改善,并且表现出剂量依赖性;此外,骨形态发生蛋白2和Smad4的基因和蛋白表达水平在槲皮素治疗后也呈现上升趋势。这表明槲皮素能够有效促进成骨分化,并通过上调骨形态发生蛋白2和Smad4的表达来改善骨质疏松症,见图8。"
| [1] García-Gómez MC, Vilahur G. Osteoporosis and vascular calcification: a shared scenario. Clin Investig Arterioscler. 2020;32(1):33-42. [2] 中国骨质疏松症流行病学调查及“健康骨骼”专项行动结果发布[J].中华骨质疏松和骨矿盐疾病杂志,2019,12(4):317-318. [3] 中华医学会骨质疏松和骨矿盐疾病分会,章振林.原发性骨质疏松症诊疗指南(2022)[J].中国全科医学,2023,26(14):1671-1691. [4] QASEEM A, HICKS LA, ETXEANDIA-IKOBALTZETA I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the american college of physicians (version 1, update alert). Ann Intern Med. 2024;177(6): eL230113. [5] GAO L, LI C, WANG Z, et al. Ligustri lucidi fructus as a traditional Chinese medicine: a review of its phytochemistry and pharmacology. Nat Prod Res. 2015;29(6):493-510. [6] 黄婉,杨耀芳.女贞子及其有效成分的药理及临床研究进展[J].现代中西医结合杂志,2003,12(7):772-774. [7] 姜斐.女贞子化学成分的提取分离鉴定及活性研究[D].南京:南京中医药大学,2010. [8] 顾闻,刘特,陈久林,等.特女贞苷对血管内皮细胞氧化损伤的作用研究[J].中国中西医结合杂志,2018,38(9):1093-1098. [9] XIA EQ, WANG BW, XU XR, et al. Microwave-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Int J Mol Sci. 2011;12(8):5319-5329. [10] QIN X, WEI Q, AN R, et al. Regulation of bone and fat balance by Fructus Ligustri Lucidi in ovariectomized mice. Pharm Biol. 2023;61(1):391-403. [11] 王佳丽,单安山,刘天阳,等.女贞子CO2超临界萃取物对断奶仔猪小肠绒毛、盲肠菌群及血常规的影响[J].东北农业大学学报, 2013,44(12):10-15. [12] 袁毅,沈丽新,潘燕.女贞子对2型糖尿病大鼠胰岛β细胞的作用及机制[J].中华中医药学刊,2019,37(1):206-208, 264. [13] ZHANG Y, LIU L, GAO J, et al. New secoiridoids from the fruits of ligustrum lucidum ait with triglyceride accumulation inhibitory effects. Fitoterapia. 2013;91:107-112. [14] DAI Y, HANG BQ, MONG QY, et al. Anti-inflammatory effect of fructus Ligustri Lucidi. Zhongguo Zhong Yao Za Zhi. 1989;14(7):431-433,448. [15] WILLEMS M. Quantitative determination of seco-iridoid glucosides from the fruits of ligustrum vulgare by HPLC. Planta Med. 1988;54(1):66-78. [16] LIN HM, YEN FL, NG LT, et al. Protective effects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J Ethnopharmacol. 2007;111(1):129-136. [17] ZHANG Y, LEUNG PC, CHE CT, et al. Improvement of bone properties and enhancement of mineralization by ethanol extract of Fructus Ligustri Lucidi. Br J Nutr. 2008;99(3):494-502. [18] LI G, ZHANG XA, ZHANG JF, et al. Ethanol extract of Fructus Ligustri Lucidi promotes osteogenesis of mesenchymal stem cells. Phytother Res. 2010;24(4):571-576. [19] ZHANG Y, DIAO TY, WANG L, et al. Protective effects of water fraction of Fructus Ligustri Lucidi extract against hypercalciuria and trabecular bone deterioration in experimentally type 1 diabetic mice. J Ethnopharmacol. 2014;158 Pt A:239-245. [20] LI P, WANG Y, YAN Q, et al. Fructus Ligustri Lucidi inhibits ferroptosis in ovariectomy‑induced osteoporosis in rats via the Nrf2/HO‑1 signaling pathway. Biomed Rep. 2023;20(2):27. [21] 刘美红,邹峥嵘.女贞子化学成分、药理作用及药动学研究进展[J].热带亚热带植物学报,2022,30(3):446-460. [22] YU WX, POON CC, ZHOU LP, et al. Oleanolic acid exerts bone anabolic effects via activation of osteoblastic 25-hydroxyvitamin D 1-alpha hydroxylase. Biomed Pharmacother. 2024;173:116402. [23] YANG Y, SHEN L, WANG P, et al. Anti-osteoporosis bioactivity evaluation in zebrafish model of raw and salt-processed Achyranthes bidentata followed by liquid chromatography-mass spectrometry analysis and correlation analysis. Biomed Chromatogr. 2023;37(12):e5742. [24] XU Y, CHEN S, YU T, et al. High-throughput metabolomics investigates anti-osteoporosis activity of oleanolic acid via regulating metabolic networks using ultra-performance liquid chromatography coupled with mass spectrometry. Phytomedicine. 2018;51:68-76. [25] CAO S, DONG XL, HO MX, et al. Oleanolic acid exerts osteoprotective effects and modulates vitamin d metabolism. Nutrients. 2018;10(2):247. [26] ZHENG H, FENG H, ZHANG W, et al. Targeting autophagy by natural product Ursolic acid for prevention and treatment of osteoporosis. Toxicol Appl Pharmacol. 2020;409:115271. [27] CHENG M, LIANG XH, WANG QW, et al. Ursolic acid prevents retinoic acid-induced bone loss in rats. Chin J Integr Med. 2019;25(3):210-215. [28] 杨然,陆远,郝昊,等.金银花环烯醚萜苷类化学成分和药理活性研究进展[J].中国中药杂志,2021,46(11):2746-2752. [29] LAO A, CHEN Y, SUN Y, et al. Transcriptomic analysis provides a new insight: oleuropein reverses high glucose-induced osteogenic inhibition in bone marrow mesenchymal stem cells via Wnt10b activation. Front Bioeng Biotechnol. 2022;10:990507. [30] SANTIAGO-MORA R, CASADO-DÍAZ A, DE CASTRO MD, et al. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: the effect on differentiation in stem cells derived from bone marrow. Osteoporos Int. 2011;22(2):675-684. [31] CHEN L, ZHANG RY, XIE J, et al. STAT3 activation by catalpol promotes osteogenesis-angiogenesis coupling, thus accelerating osteoporotic bone repair. Stem Cell Res Ther. 2021;12(1):108. [32] CHENG J, XU HY, LIU MM, et al. Catalpol promotes the proliferation and differentiation of osteoblasts induced by high glucose by inhibiting KDM7A. Diabetes Metab Syndr Obes. 2020;13:705-712. [33] LI Y, WANG MJ, LI S, et al. Effect of total glycosides from Eucommia ulmoides seed on bone microarchitecture in rats. Phytother Res. 2011; 25(12):1895-1907. [34] MARTINIAKOVA M, BABIKOVA M, OMELKA R. Pharmacological agents and natural compounds: available treatments for osteoporosis. J Physiol Pharmacol. 2020. doi: 10.26402/jpp.2020.3.01. [35] CHEN XF, LI XL, YANG M, et al. Osteoprotective effects of salidroside in ovariectomized mice and diabetic mice. Eur J Pharmacol. 2018; 819:281-288. [36] XIE B, ZHOU H, LIU H, et al. Salidroside alleviates dexamethasone-induced inhibition of bone formation via transforming growth factor-beta/Smad2/3 signaling pathway. Phytother Res. 2023;37(5):1938-1950. [37] LI F, YANG X, YANG Y, et al. Antiosteoporotic activity of echinacoside in ovariectomized rats. Phytomedicine. 2013;20(6):549-557. [38] ALI D, OKLA M, ABUElREICH S, et al. Apigenin and Rutaecarpine reduce the burden of cellular senescence in bone marrow stromal stem cells. Front Endocrinol (Lausanne). 2024;15:1360054. [39] CHOI EM. Apigenin increases osteoblastic differentiation and inhibits tumor necrosis factor-alpha-induced production of interleukin-6 and nitric oxide in osteoblastic MC3T3-E1 cells. Pharmazie. 2007;62(3):216-220. [40] SUN J, PAN Y, LI X, et al. Quercetin attenuates osteoporosis in orchiectomy mice by regulating glucose and lipid metabolism via the GPRC6A/AMPK/mTOR signaling pathway. Front Endocrinol (Lausanne). 2022;13:849544. [41] ZHENG L. Luteolin stimulates proliferation and inhibits late differentiation of primary rat calvarial osteoblast induced by high-dose dexamethasone via Sema3A /NRP1/Pleixin A1. Curr Pharm Biotechnol. 2021;22(11):1538-1545. [42] KIM TH, JUNG JW, HA BG, et al. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J Nutr Biochem. 2011;22(1):8-15. [43] YAMAGUCHI M, LEVY RM. β-Caryophyllene promotes osteoblastic mineralization, and suppresses osteoclastogenesis and adipogenesis in mouse bone marrow cultures in vitro. Exp Ther Med. 2016;12(6):3602-3606. [44] SHAN J, CHEN L, LU K. Protective effects of trans-caryophyllene on maintaining osteoblast function. IUBMB Life. 2017;69(1):22-29. [45] PUJIA A, RUSSO C, MAUROTTI S, et al. Bergamot polyphenol fraction exerts effects on bone biology by activating ERK 1/2 and Wnt/β-catenin pathway and regulating bone biomarkers in bone cell cultures. Nutrients. 2018;10(9):1305. [46] HONG G, HE X, SHEN Y, et al. Chrysosplenetin promotes osteoblastogenesis of bone marrow stromal cells via Wnt/β-catenin pathway and enhances osteogenesis in estrogen deficiency-induced bone loss. Stem Cell Res Ther. 2019;10(1):277. [47] LIU C, WANG L, ZHU R, et al. Rehmanniae Radix Preparata suppresses bone loss and increases bone strength through interfering with canonical Wnt/β-catenin signaling pathway in OVX rats. Osteoporos Int. 2019;30(2):491-505. [48] ZHU Y, WANG Y, JIA Y, et al. Catalpol promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10(1):37.
[49] LIU H, GUO Y, ZHU R, et al. Fructus Ligustri Lucidi preserves bone quality through induction of canonical Wnt/β-catenin signaling pathway in ovariectomized rats. Phytother Res. 2021;35(1):424-441. [50] PAN FF, SHAO J, SHI CJ, et al. Apigenin promotes osteogenic differentiation of mesenchymal stem cells and accelerates bone fracture healing via activating Wnt/β-catenin signaling. Am J Physiol Endocrinol Metab. 2021;320(4):E760-E771. [51] ONO T, HAYASHI M, SASAKI F, et al. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2. [52] LIU FL, CHEN CL, LEE CC, et al. The simultaneous inhibitory effect of niclosamide on RANKL-induced osteoclast formation and osteoblast differentiation. Int J Med Sci. 2017;14(9):840-852. [53] KANG JY, KANG N, YANG YM, et al. The role of Ca2+-NFATc1 signaling and its modulation on osteoclastogenesis. Int J Mol Sci. 2020;21(10):3646. [54] PARK B, SONG HS, KWON JE, et al. Effects of Salvia miltiorrhiza extract with supplemental liquefied calcium on osteoporosis in calcium-deficient ovariectomized mice. BMC Complement Altern Med. 2017; 17(1):545. [55] ZHENG H, QI S, CHEN C. Salidroside improves bone histomorphology and prevents bone loss in ovariectomized diabetic rats by upregulating the OPG/RANKL ratio. Molecules. 2018;23(9):2398. [56] LI F, YANG Y, ZHU P, et al. Echinacoside promotes bone regeneration by increasing OPG/RANKL ratio in MC3T3-E1 cells. Fitoterapia. 2012; 83(8):1443-1450. [57] LIU H, ZHAO A, HUANG Y, et al. Efficacy and mechanisms of oleuropein in postmenopausal osteoporosis. Comput Math Methods Med. 2022; 2022:9767113. [58] ZHANG Y, LIU X, LI Y, et al. Aucubin slows the development of osteoporosis by inhibiting osteoclast differentiation via the nuclear factor erythroid 2-related factor 2-mediated antioxidation pathway. Pharm Biol. 2021;59(1):1556-1565. [59] BELLEZZA I, GIAMBANCO I, MINELLI A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721-733. [60] TONELLI C, CHIO IIC, TUVESON DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727-1745. [61] ZHANG Z, QU J, ZHENG C, et al. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis. Cell Death Dis. 2018;9(2):83. [62] RYTER SW. Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation. Cells. 2021;10(3):515. [63] PUENTES-PARDO JD, MORENO-SANTUAN S, CARAZO Á, et al. Heme oxygenase-1 in gastrointestinal tract health and disease. Antioxidants (Basel). 2020;9(12):1214. [64] VIJAYAN V, WAGENER FADTG, IMMENSCHUH S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem Pharmacol. 2018;153:159-167. [65] WANG YF, CHANG YY, ZHANG XM, et al. Salidroside protects against osteoporosis in ovariectomized rats by inhibiting oxidative stress and promoting osteogenesis via Nrf2 activation. Phytomedicine. 2022;99:154020. [66] WANG K, ZHOU C, LI L, et al. Aucubin promotes bone-fracture healing via the dual effects of anti-oxidative damage and enhancing osteoblastogenesis of hBM-MSCs. Stem Cell Res Ther. 2022;13(1):424. [67] SUH KS, CHON S, CHOI EM. Luteolin alleviates methylglyoxal-induced cytotoxicity in osteoblastic MC3T3-E1 cells. Cytotechnology. 2016;68(6):2539-2552. [68] MCGONNELL IM, GRIGORIADIS AE, LAM EW, et al. A specific role for phosphoinositide 3-kinase and AKT in osteoblasts? Front Endocrinol (Lausanne). 2012;3:88. [69] YEON JT, KIM KJ, SON YJ, et al. Idelalisib inhibits osteoclast differentiation and pre-osteoclast migration by blocking the PI3Kδ-Akt-c-Fos/NFATc1 signaling cascade. Arch Pharm Res. 2019;42(8):712-721. [70] HAN SY, KIM YK. Berberine suppresses RANKL-induced osteoclast differentiation by inhibiting c-Fos and NFATc1 expression. Am J Chin Med. 2019;47(2):439-455. [71] BAKKER AD, GAKES T, HOGERVORST JM, et al. Mechanical stimulation and IGF-1 enhance mRNA translation rate in osteoblasts via activation of the AKT-mTOR pathway. J Cell Physiol. 2016;231(6):1283-1290. [72] JIANG T, GU H, WEI J. Echinacoside inhibits osteoclast function by down-regulating PI3K/AKT/c-fos to alleviate osteolysis caused by periprosthetic joint infection. Front Pharmacol. 2022;13:930053. [73] LI S, CUI Y, LI M, et al. Acteoside derived from cistanche improves glucocorticoid-induced osteoporosis by activating PI3K/AKT/mTOR pathway. J Invest Surg. 2023;36(1):2154578. [74] GONG W, ZHANG N, CHENG G, et al. Rehmannia glutinosa libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR pathway in streptozotocin-induced diabetic rats. Int J Mol Sci. 2019;20(16):3964. [75] LIANG G, ZHAO J, DOU Y, et al. Mechanism and experimental verification of luteolin for the treatment of osteoporosis based on network pharmacology. Front Endocrinol (Lausanne). 2022;13:866641. [76] YU D, HUANG C, JIANG C, et al. Features of a simvastatin-loaded multi-layered co-electrospun barrier membrane for guided bone regeneration. Exp Ther Med. 2021;22(1):713. [77] Muñoz J, Akhavan NS, Mulliins AP, et al. Macrophage polarization and osteoporosis: a review. Nutrients. 2020;12(10):2999. [78] WANG Z, BAO HW, XU YJ. Cnidium lactone prevents bone loss in an ovariectomized rat model through the estrogen-α/BMP-2/Smad signaling pathway. J Gene Med. 2020;22(8):e3198. [79] LI Y, HU W, HAN G, et al. Involvement of bone morphogenetic protein-related pathways in the effect of aucubin on the promotion of osteoblast differentiation in MG63 cells. Chem Biol Interact. 2018; 283:51-58. [80] LI Y, ZHANG Y, ZHANG X, et al. Aucubin exerts anti-osteoporotic effects by promoting osteoblast differentiation. Aging (Albany NY). 2020;12(3):2226-2245. [81] 郑红,唐薇,角建林,等.槲皮素通过促进成骨分化治疗去势骨质疏松症大鼠的分子机制[J].中药药理与临床,2017,33(5):16-20. [82] MA Z, TANG X, CHEN Y, et al. Epimedii folium and ligustri lucidi fructus promote osteoblastogenesis and inhibit osteoclastogenesis against osteoporosis via acting on osteoblast-osteoclast communication. Oxid Med Cell Longev. 2023;2023:7212642. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [3] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [4] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [5] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [6] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [7] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [8] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [9] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [10] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [11] | Zhang Haojun, Li Hongyi, Zhang Hui, Chen Haoran, Zhang Lizhong, Geng Jie, Hou Chuandong, Yu Qi, He Peifeng, Jia Jinpeng, Lu Xuechun. Identification and drug sensitivity analysis of key molecular markers in mesenchymal cell-derived osteosarcoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1448-1456. |
| [12] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [13] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [14] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [15] | Li Yueyao, Zhang Min, Yang Jiaju. Cistanoside A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1144-1151. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||