Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (17): 3632-3640.doi: 10.12307/2025.634
Previous Articles Next Articles
Diabetic nephropathy model: animal model, two-dimensional cell simulation and three-dimensional organoid model
Qian Zuping1, 2, Chen Yong2, Ran Yan2, Da Jingjing2, Zha Yan1, 2
- 1Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2Department of Nephrology, Guizhou Provincial People’s Hospital, Guiyang 550002, Guizhou Province, China
-
Received:2024-06-06Accepted:2024-07-22Online:2025-06-18Published:2024-11-02 -
Contact:Zha Yan, MD, Chief physician, Professor, Doctoral supervisor, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Department of Nephrology, Guizhou Provincial People’s Hospital, Guiyang 550002, Guizhou Province, China -
About author:Qian Zuping, Master candidate, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Department of Nephrology, Guizhou Provincial People’s Hospital, Guiyang 550002, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 82360148 (to ZY); Science and Technology Department of Guizhou Province, No. Guizhou Family Synthetic Fruit [2023] Major 010 (to ZY); Guizhou High-level Innovative Talents Project, No. Guizhou Science Cooperation Platform Talents [2018] 5636-2 (to ZY); Guizhou Science and Technology Plan Project, No.Guizhou Science Cooperation Platform Talent [2020] 2201 (to ZY); Talent Fund of Guizhou Provincial People’s Hospital, No. [2023]-8 (to CY); Guizhou Science and Technology Plan Project, No. Guizhou Science Cooperation Basis-ZK[2021]381 (to RY); Guizhou Science and Technology Plan Project, No. Guizhou Science Cooperation Basis-ZK[2023]219 (to ZY)
CLC Number:
Cite this article
Qian Zuping, Chen Yong, Ran Yan, Da Jingjing, Zha Yan. Diabetic nephropathy model: animal model, two-dimensional cell simulation and three-dimensional organoid model [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3632-3640.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
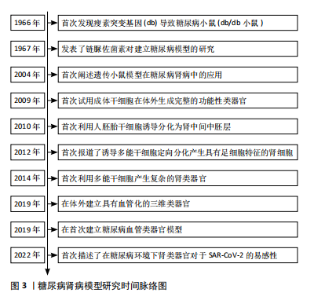
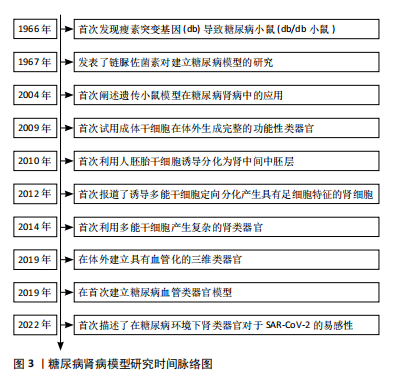
通过检索相关文献,将糖尿病肾病模型研究时间线进行了总结,目前最常用的糖尿病肾病小鼠模型源于1966年HUMMEL等[7]发现的由于瘦素受体基因(db)突变导致的糖尿病小鼠模型;利用链脲佐菌素诱导糖尿病肾病模型的化学诱导法和遗传基因背景下的糖尿病肾病模型也随后被JUNOD等[8]和ALLEN等[9]研究报道;2009年,SATO等[10]首次报道了利用成体干细胞在体外生成具有功能的肠道类器官,为之后研究类器官奠定了基础;2010年,LIN 等[11]利用人胚胎干细胞诱导分化为肾中间中胚层,为之后研究肾类器官奠定了基础;2012年,SONG等 [12] 首次报道了利用多能干细胞定向诱导分化为具有足细胞特征的肾细胞;2014年,TAGUCHI等[13]报道了多能干细胞产生复杂的肾类器官;2019年,LOW等[14]报道在体外产生了具有血管化的肾类器官;同时,WIMMER等[15]报道了首次利用血管类器官建立糖尿病血管类器官模型;2022年,GARRETA等[16]首次描述了将肾类器官培养在糖尿病环境中,模拟了部分糖尿病肾病的病理改变,见图3。"
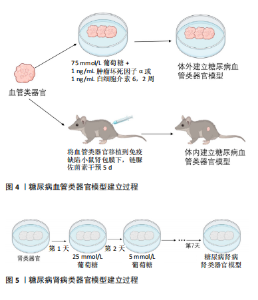
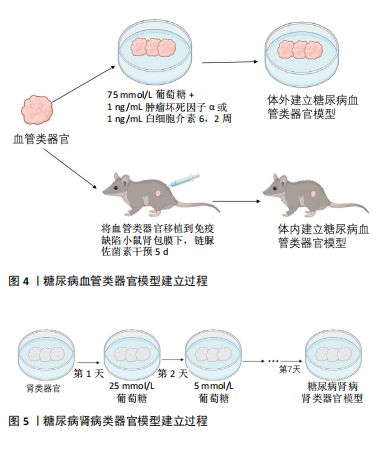
2.1 肾类器官 近年来,兴起的“类器官”为研究糖尿病疾病模型提供了一种新的、具有潜力的模型。类器官是由干细胞产生的、能够在体外自发形成组织的3D培养系统,具有稳定的遗传背景,可以再现器官结构和功能[17]。三维培养的肾类器官模拟了人体复杂的组织结构和一些简单的功能,它们的发展标志着二维细胞培养的巨大飞跃,二维细胞培养和动物模型对研究肾脏疾病均做出了巨大贡献,然而这些模型往往不能完全复制人体器官的生理和病理过程[18];并且由于物种之间的差异影响,限制了将研究结果转化为临床应用[19];此外,由于人体器官生理和病理过程的复杂性,依靠单纯的二维细胞培养不能满足细胞之间错综复杂的相互作用关系,一个结构化的三维类器官为解决这些问题提供了一种可能[20-21]。 最早有关肾类器官的报道是通过不添加任何细胞因子的血清自分化而产生胚胎后肾以及少量的肾祖细胞[11]。随后,经过科研人员的不断探索[22],将人多能干细胞诱导分化形成包含足细胞、近端小管上皮细胞、远端小管上皮细胞及血管内皮细胞等多种肾脏固有细胞三维肾脏类器官[23]。尽管不同的诱导方法在诱导时间、复杂性和可扩展性方面存在差异,但最终的类器官在组织复杂性和成熟度方面是相似的,能提供多种细胞间相互作用及协作发育并形成具有一定结构和功能的迷你肾,能更好地模拟人肾脏发育及生理病理状态,并且已经有许多研究利用肾类器官模拟肾脏疾病模型,如遗传性多囊肾[24-25]、急性肾损伤和肾癌等[26-28]。同时肾类器官也是未来建立糖尿病肾病模型的潜在工具,来自不同个体的多能干细胞衍生的肾脏类器官包含人类遗传异质性,可以分析糖尿病肾病患者个体间的差异性;还能从糖尿病肾病的动物模型在人类背景下进行验证,而不需要患者的肾组织样本;同时还能在类器官中进行高通量药物筛选和治疗评估[29]。肾类器官不仅在肾脏发育、疾病建模及药物筛选方面具有重要作用,而且,根据最新的研究报道,利用免疫逃避诱导的肾类器官有望用于临床移植为临床肾移植带来了一定的希望[30]。 2.2 糖尿病肾病类器官模型 研究显示,由人多能干细胞诱导分化的含有内皮细胞及周细胞的血管类器官,暴露在高血糖或炎症细胞因子条件下能较好地模拟糖尿病对血管的损伤,出现与临床患者相似的病理改变即血管基底膜增厚[15](如图4)。由于糖尿病肾病是糖尿病晚期微血管并发症之一,因此该研究为后续建立糖尿病肾病类器官模型奠定基础。近期的一项研究表明,将人多能干细胞源肾类器官放在体外建立的糖尿病环境中(以24 h为一周期,在奇数天加入5 mmol/L葡萄糖,在偶数天加入25 mmol/L 葡萄糖),与模拟健康个体的葡萄糖恒定为5 mmol/L的肾类器官相比,糖尿病环境下的肾类器官出现了纤维化等糖尿病肾病病理特征以及改变了肾类器官的代谢[29](如图5)。"
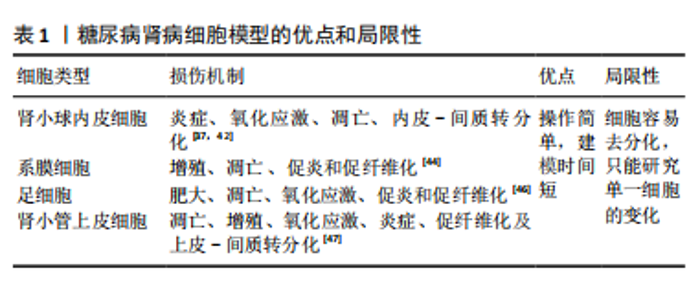
目前,一方面,虽然肾类器官仍处于胎肾阶段,如何促进其成熟并具备功能是未来利用类器官研究糖尿病肾病的关键因素[23];另一方面,肾脏类器官的有限寿命将是糖尿病肾病研究需要克服的一个重要限制,尽管已经有研究报道可以将类器官培养至少60多天[14],但是这些类器官在培养30多天后会发生纤维化和脱靶细胞的扩张[23],这种自发性纤维化也会使发现糖尿病肾病的晚期纤维化变得更加困难[31]。肾类器官模拟糖尿病肾病的体外培养条件,如葡萄糖浓度、作用时间及是否要加入炎症因子等因素仍需探索。 尽管以上提到了肾脏类器官的一些限制,然而,随着对类器官的研究,不断有研究人员提出一些解决办法,如有研究人员发现微环境的流变学和力学特性对类器官培养及成熟至关重要[32]。因此,有许多研究人员将生物材料与类器官相结合,通过改变培养类器官的微环境,从而促进了类器官的成熟,如水凝胶[33]、肾脱细胞细胞外基质水凝胶和流体动力学等[34-35]。肾脏微血管的损伤对于糖尿病肾病发生发展过程具有重要意义,最近有研究报道他们创建了一个可灌注的、血管化的肾类器官芯片模型,证明了右旋糖酐和红细胞可以通过大血管、微血管网络灌注到肾类器官中足细胞簇中,这增加了建立糖尿病肾病类器官模型的可行性[36]。在糖尿病肾病早期,系膜细胞的改变对于疾病的发生发展具有重要作用[37],最新的研究报道通过CHIR99021、SAG、SB431542、IL-1b和RA选择性地诱导了人多能干细胞来源的肾间充质干细胞,从而诱导分化出系膜细胞和erythropoietin(EPO)细胞[38],这为研究糖尿病肾病早期系膜细胞与足细胞及小管细胞之间的相互作用机制提供了一个新的可能。此外,肾类器官缺乏免疫细胞,而免疫细胞在糖尿病肾病中的作用不容忽视,尽管目前的手段无法在肾类器官中诱导出免疫细胞,但最近有研究人员将人诱导多能干细胞衍生的肾类器官与人外周血单个核细胞共培养,观察两者的相互作用,为在肾脏类器官中引入免疫细胞研究肾脏疾病及药物相互的作用创造了一个更现实的模型[39]。同时,有研究人员采用人单核细胞和人多能干细胞诱导分化的巨噬细胞利用transwell共培养的方法分化人多能干细胞,发现这种方法导致肾类器官数量更多、形态结构更好,为提高肾脏类器官模型的实用性提供了一种新的途径[40]。说明免疫细胞不仅在肾脏疾病中不可或缺,在肾脏发育过程中也具有重要作用。 在利用肾类器官建立糖尿病肾病模型时,一方面不仅可以通过改变体外培养条件来建立,也可以尝试利用糖尿病肾病患者的细胞来建立肾类器官,以概括糖尿病的表观遗传易感性,还可以从基因修饰的人多能干细胞中诱导分化,用于测试特定基因的功能,如缺乏内皮型一氧化氮合酶从而建立糖尿病肾病小鼠模型,同样可以通过基因编辑的方式建立糖尿病肾病肾类器官模型;另一方面,可以将肾类器官移植到小鼠肾包膜下,用链脲佐菌素诱导携带肾类器官的小鼠成为糖尿病模型,使肾类器官暴露在糖尿病环境下,建立糖尿病肾病模型;前文提到的糖尿病血管类器官模型利用这种方法建立了与体外干预类似的病理变化[41]。总之,肾类器官的出现为糖尿病肾病模型提供了一个新的、具有巨大潜力的平台。 2.3 糖尿病肾病细胞模型 糖尿病肾病体外模型包括二维肾源细胞系培养模型和新兴的三维肾类器官模型,大量基于二维肾源细胞系培养模型的研究揭示了糖尿病肾病损伤机制,同时也为药物筛选提供了便捷的平台。 肾小球的损伤在糖尿病肾病的病理变化过程中起着重要作用,主要包括内皮细胞、足细胞和系膜细胞等固有细胞的损伤[37]。肾小管内皮细胞在肾小球滤过及尿液形成中具有重要作用,在高血糖条件下容易引发细胞氧化应激、炎症及细胞凋亡,最近的研究发现肾小球内皮细胞中内皮-间质转分化在糖尿病肾病进程中具有重要作用[42]。肾小球系膜细胞对调节肾小球血流动力学、维持细胞外基质稳态和吞噬凋亡细胞方面发挥着重要作 用[43]。持续高血糖直接刺激系膜细胞导致系膜细胞功能障碍,如系膜细胞过度增殖、肥大、分泌促炎、促纤维化、促凋亡因子并激活诸多信号通路,分泌过量的细胞外基质,造成系膜基质增加和基底膜增厚,导致肾小球硬 化[44]。基于系膜细胞系二维培养的研究表明:在高糖培养环境下系膜细胞会显著增殖,核因子κB、转化生长因子β、TRPC6和纤维连接蛋白的表达亦上调,高糖培养也会诱导系膜细胞凋亡[41]。足细胞的损伤和丢失是糖尿病肾病早期重要的病理标志,在糖尿病环境下培养的足细胞由于高糖、生长因子、脂肪酸、血管紧张素Ⅱ、转化生长因子β、激素和机械拉伸等因素,导致足细胞损伤、凋亡以及修复和再生能力受到限制,使足细胞数量减少,加速糖尿病肾病的发展[45-46]。虽然许多关于糖尿病肾病的研究都集中在肾小球上,但越来越多的证据表明肾小管在疾病进展中的重要性[47]。因此,将二维培养的小管细胞暴露于模拟糖尿病肾病的病理生理介质,包括高糖[48]、高脂和炎症因子及糖化蛋白和血管紧张素Ⅱ方式[49-50],已被广泛用于糖尿病肾病的体外模型探索肾小管细胞损伤的机制(表1)。"
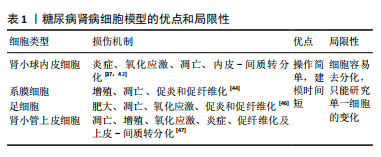
尽管有研究人员提出二维细胞系培养系统不能提供多种细胞之间的相互作用且不能形成肾脏样结构[51]、也不能准确地模拟肾脏病理生理过程[52-53],且原代细胞长期培养或扩增较困难,它会在几天内去分化并衰老[54]。然而,最近许多研究人也开始关注在糖尿病肾病中肾脏各种细胞之间的相互作用[37,55-56],以及由于二维的肾源细胞培养的操作简单和建模时间周期短依然成为了目前研究中建立糖尿病肾病体外模型的首选。 2.4 糖尿病肾病动物模型 糖尿病并发症动物模型联盟提出了理想的糖尿病肾病小鼠模型的3个标准:高血糖情况下进行性肾功能不全、蛋白尿及特征性病理改变(包括电镜下基底膜增厚、系膜基质扩张或结节性系膜硬化、小管间质纤维化和小动脉透明质病变)[57]。一个理想的疾病模型对于探索疾病的发病机制具有重要作用,目前根据造模的原理不同,糖尿病肾病动物模型可以分为转基因型、自发型、诱导型及中医结合病证型。 2.4.1 转基因糖尿病肾病模型 OVE26小鼠是在胰腺β细胞中过表达钙调蛋白,从而导致胰岛素分泌不足进而导致系膜基质增加、肾小球硬化和足细胞数量减少[58]。有研究发现OVE26小鼠肾脏损伤程度与小鼠遗传背景及性别有关,FVB遗传背景下及雌性OVE26小鼠肾脏损伤更严重[58-59]。有研究人员为了更快速地产生糖尿病肾病模型,在OVE26的背景上对2 月龄小鼠进行单侧肾切除,从而加速了肾脏的损伤[60]。有研究报道内皮型一氧化氮合酶(eNOS)的缺乏会加速糖尿病小鼠肾脏的损伤,因此研究人员把内皮型一氧化氮合酶基因敲除(eNOS KO)发现小鼠出现明显的蛋白尿、高血压和肾小球系膜溶解[61]。作为适合研究2型糖尿病引起糖尿病肾病的Zucker糖尿病性脂肪(ZDF)大鼠在12周龄时出现明显的高血糖,肾脏无病理变化;在22周龄时尿白蛋白与肌酐比显著增加,肾小球出现微动脉瘤;40周龄时,肾脏出现不可逆损伤包括肾小球萎缩、微动脉瘤和硬化[62]。含有天然胰岛素抵抗基因的黑褐色、短尾(BTBR)小鼠与ob/ob瘦素突变小鼠杂交的子代小鼠在8周时出现明显的高血糖和蛋白尿及肾脏肾小球系膜基质积累,在22周龄时出现糖尿病肾病相对严重的病理改变(包括弥漫性、局灶性结节性肾小球硬化及广泛的系膜基质扩张)[63]。见表2。"
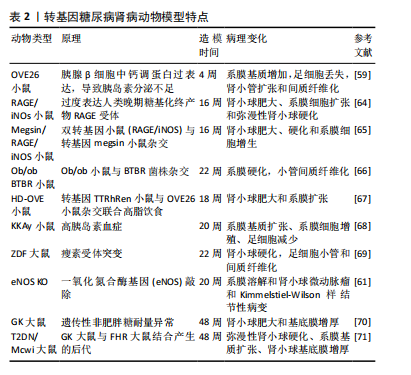
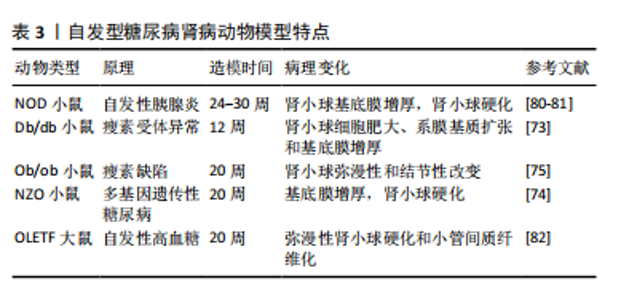
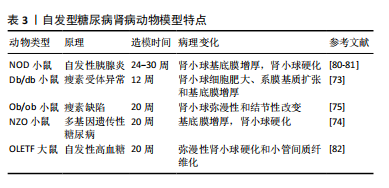
2.4.2 自发型糖尿病肾病模型 目前常用的糖尿病肾病模型为db/db小鼠,db/db小鼠编码瘦素受体的糖尿病(db)基因发生突变,使瘦素受体(ObRb)的长异构体完全缺乏瘦素信号,从而使体内的瘦素循环过表达[72]。在db/db小鼠肾脏中观察到毛细血管基底膜增厚、外周毛细血管袢塌陷及系膜弥漫性扩张等早期糖尿病肾病表现[73]。ob/ob小鼠是另一种模拟糖尿病的肥胖小鼠,在ob/ob小鼠中完全缺乏瘦素产生,ob/ob小鼠的肾脏病理变化主要为肾小球弥漫性和结节性硬化[74],研究报道,ob/ob小鼠的糖尿病肾病病变较db/db小鼠相对较轻[75]。因此这两种糖尿病肾病模型适合做为研究早期糖尿病肾病发病机制的模型。非肥胖型糖尿病(nonobese diabetic,NOD)小鼠在糖尿病发病后血浆肌酐和血尿素氮浓度逐渐升高,随后出现蛋白尿、肾系膜增生和硬化[76],该模型小鼠的缺点在于遗传背景复杂,且由于是自身免疫性疾病,出现高血糖的时间不一致[74,77]。KKAy小鼠是一种高胰岛素血症小鼠模型,仅表现出轻微的肾脏病理,包括基底膜增厚和弥漫性肾小球硬化,可作为研究早期糖尿病肾病的模型[78]。新西兰肥胖鼠(NZO)是研究自身免疫性糖尿病的潜在研究模型,它具有导致肥胖和2型糖尿病的多基因遗传背景,在12个月时肾脏表现出糖尿病和狼疮肾病的光镜特征:肾小球增殖、系膜沉积、轻度基底膜增厚、肾小球硬化、一些肾小球中的嗜酸性结节、肾小球小动脉偶尔透明样变以及小动脉炎症愈合,它为研究自身免疫、肥胖和糖尿病之间的关系提供了模型[79]。见表3。"
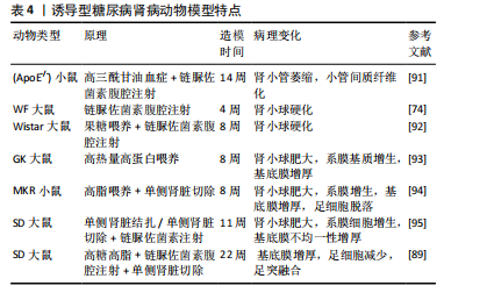
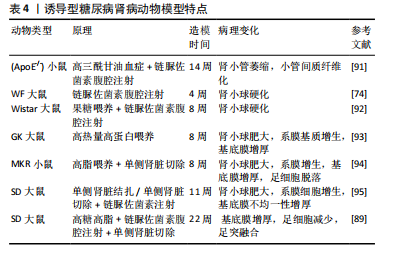
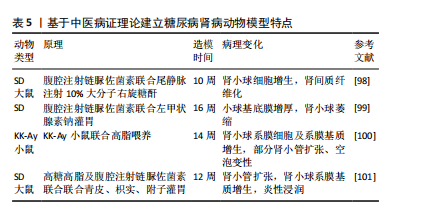
2.4.3 诱导型糖尿病肾病模型 链脲佐菌素(STZ)是化学诱导法制备糖尿病肾病模型最常用的诱导剂,具有胰腺毒性,会破坏胰腺细胞[83],链脲佐菌素诱导的糖尿病动物已成为研究1型糖尿病及其并发症的主要实验模型[84]。利用链脲佐菌素诱导1型糖尿病主要有两种方式,一种是单次大剂量,一种是多次小剂量,多次小剂量链脲佐菌素诱导小鼠引起高血糖的发病机制和形态学变化方面更类似于1型糖尿病,并且肾脏表现出更接近糖尿病肾病的病理特征且药物毒性更小,小鼠存活率高[85-86],但建模时间更长[87]。由于2型糖尿病患者大多数属于肥胖型患者,多有高脂血症,高脂高糖共同促进了肾脏的损伤,因此,有研究利用高脂饲料喂养联合腹腔注射低剂量链脲佐菌素来制备糖尿病肾病大鼠模型,病理染色显示该模型除了早期的肾脏变化,还出现部分肾小球硬化、肾小管部分出现细胞肥大以及管腔变窄[88],但该方法的建模时间周期较长,要在12周时肾脏才开始出现病理改变[87]。有研究人员应用“三联法”:高糖高脂喂养联合腹腔注射小剂量链脲佐菌素联合单侧肾脏切除,从而达到缩短造模时间的目的[89-90]。见表4。 2.4.4 中医病证结合建立糖尿病肾病模型 中医认为糖尿病肾病可归属于“消渴”“水肿”“尿浊”“溺毒”等范畴,辨证施治对疾病有一定疗效[96],因此建立病证结合动物模型对于中医治疗糖尿病肾病具有重要作用。病证结合动物模型是指在中医药理论与现代医学理论及实验动物学结合下构建疾病模型,使模型同时具备西医疾病与中医证候特征的优势,从而观察中医证候对疾病的影响[97]。中医认为,血瘀证始终贯穿糖尿病肾病病程,既是该病的"
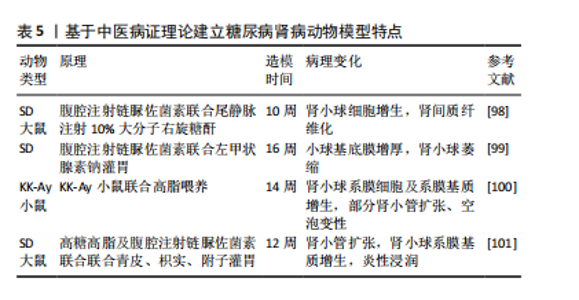
产物又是加重肾损伤的重要因素,因此有研究人员使用链脲佐菌素建立糖尿病肾病模型之后在尾静脉中注射10%的大分子右旋糖酐,成功建立了血瘀证糖尿病肾病模型,并且与糖尿病肾病组相比,血瘀证糖尿病肾病组的肾脏损伤更严重[98]。同时,阴虚在糖尿病肾病疾病进展中也起着不可忽视的作用,因此,有研究报道通过在糖尿病肾病动物模型上再结合左甲状腺素钠水溶液灌胃,从而建立了糖尿病肾病阴虚证模型,该模型与糖尿病肾病组相比肾脏损伤更严重[99]。高云霄等[100]根据“病证同源模型”理论,在自发性糖尿病模型KK-Ay小鼠基础上,仅给予高脂饮食造模,观察气阴两虚证候演变,从而建立了糖尿病肾病气阴两虚证动物模型,并且该方法方便简单。陈鹏德等[101]根据青皮、枳实有疏肝破气之功,过用该药可使正气耗散;附子入肾经,大热,过用可耗伤肾阴,用青皮、枳实、附子灌胃构建糖尿病肾病阴虚证病证结合模型,最终部分形成气阴两虚证糖尿病肾脏疾病基本证候特征,并且证明了大鼠中标志性肠道菌群,可一定程度反映病证结合模型的基本特征。见表5。"
| [1] TINAJERO MG, MALIK VS. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol Metab Clin North Am. 2021;50(3):337-355. [2] YU MG, GORDIN D, FU J, et al. Protective Factors and the Pathogenesis of Complications in Diabetes. Endocr Rev. 2024;45(2):227-252. [3] GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020; 395(10225):709-733. [4] MOHANDES S, DOKE T, HU H, et al. Molecular pathways that drive diabetic kidney disease. J Clin Invest. 2023;133(4):e165654. [5] American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-80. [6] GHEITH O, FAROUK N, NAMPOORY N, et al. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2015;5(1):49-56. [7] HUMMEL KP, DICKIE MM, COLEMAN DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127-1128. [8] JUNOD A, LAMBERT AE, ORCI L, et al. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967;126(1):201-205. [9] ALLEN TJ, COOPER ME, LAN HY. Use of genetic mouse models in the study of diabetic nephropathy. Curr Diab Rep. 2004;4(6):435-440. [10] SATO T, VRIES RG, SNIPPERT HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262-265. [11] LIN SA, KOLLE G, GRIMMOND SM, et al. Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and putative renal progenitors. Stem Cells Dev. 2010;19(10): 1637-1648. [12] SONG B, SMINK AM, JONES CV, et al. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7(9):e46453. [13] TAGUCHI A, KAKU Y, OHMORI T, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014; 14(1):53-67. [14] LOW JH, LI P, CHEW EGY, et al. Generation of Human PSC-Derived Kidney Organoids with Patterned Nephron Segments and a De Novo Vascular Network. Cell Stem Cell. 2019;25(3):373-387.e9. [15] WIMMER RA, LEOPOLDI A, AICHINGER M, et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019; 565(7740):505-510. [16] GARRETA E, PRADO P, STANIFER ML, et al. A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells. Cell Metab. 2022;34(6): 857-873.e9. [17] HUCH M, KNOBLICH JA, LUTOLF MP, et al. The hope and the hype of organoid research. Development. 2017;144(6):938-941. [18] SONG SS, PARK HJ, KIM YK, et al. Revolutionizing biomedical research: The imperative need for heart-kidney-connected organoids. APL Bioeng. 2024;8(1):010902. [19] SCHUTGENS F, CLEVERS H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu Rev Pathol. 2020;15:211-234. [20] MCCRACKEN KW, CATÁ EM, CRAWFORD CM, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516(7531):400-404. [21] ADER M, TANAKA EM. Modeling human development in 3D culture. Curr Opin Cell Biol. 2014;31:23-28.
[22] LONG HY, QIAN ZP, LAN Q, et al. Human pluripotent stem cell-derived kidney organoids: Current progress and challenges. World J Stem Cells. 2024;16(2):114-125. [23] NISHINAKAMURA R. Human kidney organoids: progress and remaining challenges. Nat Rev Nephrol. 2019;15(10):613-624. [24] FACIOLI R, LOJUDICE FH, ANAUATE AC, et al. Kidney organoids generated from erythroid progenitors cells of patients with autosomal dominant polycystic kidney disease. PloS one. 2021;16(8):e0252156. [25] TRAN T, SONG CJ, NGUYEN T, et al. A scalable organoid model of human autosomal dominant polycystic kidney disease for disease mechanism and drug discovery. Cell Stem Cell. 2022;29(7):1083-1101.e7. [26] DIGBY JLM, VANICHAPOL T, PRZEPIORSKI A, et al. Evaluation of cisplatin-induced injury in human kidney organoids. Am J Physiol Renal Physiol. 2020;318(4):F971-F978. [27] NGUYEN L, WRUCK W, ERICHSEN L, et al. The Nephrotoxin Puromycin Aminonucleoside Induces Injury in Kidney Organoids Differentiated from Induced Pluripotent Stem Cells. Cells. 2022;11(4):635. [28] LI Z, XU H, YU L, et al. Patient-derived renal cell carcinoma organoids for personalized cancer therapy. Clin Transl Med. 2022;12(7):e970. [29] OISHI H, TABIBZADEH N, MORIZANE R. Advancing preclinical drug evaluation through automated 3D imaging for high-throughput screening with kidney organoids. Biofabrication. 2024;16(3): 10.1088/1758-5090/ad38df. [30] GAYKEMA LH, VAN NIEUWLAND RY, LIEVERS E, et al. T-Cell Mediated Immune Rejection of Beta-2-Microglobulin Knockout Induced Pluripotent Stem Cell-Derived Kidney Organoids. Stem Cells Transl Med. 2024;13(1):69-82. [31] BEYDAG-TASÖZ BS, YENNEK S, GRAPIN-BOTTON A. Towards a better understanding of diabetes mellitus using organoid models. Nat Rev Endocrinol. 2023;19(4):232-248. [32] KIM JW, NAM SA, YI J, et al. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv Mater. 2022;9(15):e2103526. [33] NERGER BA, SINHA S, LEE NN, et al. 3D Hydrogel Encapsulation Regulates Nephrogenesis in Kidney Organoids. Adv Mater. 2024;36(14): e2308325. [34] GARRETA E, MOYA-RULL D, MARCO A, et al. Natural Hydrogels Support Kidney Organoid Generation and Promote In Vitro Angiogenesis. Adv Mater. 2024:e2400306. doi: 10.1002/adma.202400306. [35] HOMAN KA, GUPTA N, KROLL KT, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019;16(3): 255-262. [36] KROLL KT, HOMAN KA, UZEL SGM, et al. A perfusable, vascularized kidney organoid-on-chip model. Biofabrication. 2024;16(4). doi: 10.1088/1758-5090/ad5ac0. [37] HU S, HANG X, WEI Y, et al. Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review. Cell Commun Signal. 2024;22(1):136. [38] TSUJIMOTO H, HOSHINA A, MAE SI, et al. Selective induction of human renal interstitial progenitor-like cell lineages from iPSCs reveals development of mesangial and EPO-producing cells. Cell Rep. 2024;43(2):113602. [39] SHANKAR AS, TEJEDA-MORA H, DU Z, et al. Interactions of the Immune System with Human Kidney Organoids. Transpl Int. 2024;37:12468. [40] PECKSEN E, TKACHUK S, SCHRöDER C, et al. Monocytes prevent apoptosis of iPSCs and promote differentiation of kidney organoids. Stem Cell Res Ther. 2024;15(1):132. [41] FANG Q, MA R Y, HE YH, et al. Effects of ferulic acid on inflammation and autophagy levels in glomerular mesangial cells induced by high glucose. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022;38(5):453-457. [42] WU M, HAO Y, WU X, et al. SirT7-mediated transcription of fascin in hyperglycemic glomerular endothelial cells contributes to EndMT in diabetic nephropathy. Acta Biochim Biophys Sin (Shanghai). 2024; 56(4):586-596. [43] ZHANG M, JIN Y, GUO X, et al. Resveratrol protects mesangial cells under high glucose by regulating the miR-1231/IGF1/ERK pathway. Environ Toxicol. 2024;39(4):2326-2339. [44] YU J, LI C, MA L, et al. Transient receptor potential canonical 6 knockdown ameliorated diabetic kidney disease by inhibiting nuclear factor of activated T cells 2 expression in glomerular mesangial cells. Ren Fail. 2022;44(1):1780-1790. [45] BENZING T, SALANT D. Insights into Glomerular Filtration and Albuminuria. N Engl J Med. 2021;384(15):1437-1446. [46] YANG C, ZHANG Z, LIU J, et al. Research progress on multiple cell death pathways of podocytes in diabetic kidney disease. Mol Med. 2023;29(1):135. [47] SLYNE J, SLATTERY C, MCMORROW T, et al. New developments concerning the proximal tubule in diabetic nephropathy: in vitro models and mechanisms. Nephrol Dial Transplant. 2015:30 Suppl 4: iv60-7. [48] KUNDU S, GHOSH S, SAHU BD. Scopoletin alleviates high glucose-induced toxicity in human renal proximal tubular cells via inhibition of oxidative damage, epithelial-mesenchymal transition, and fibrogenesis. Mol Biol Rep. 2024;51(1):620. [49] WANG CH, SURBHI, GORAYA S, et al. Fatty acids and inflammatory stimuli induce expression of long-chain acyl-CoA synthetase 1 to promote lipid remodeling in diabetic kidney disease. J Biol Chem. 2024;300(1):105502. [50] CHANG J, YAN J, LI X, et al. Update on the Mechanisms of Tubular Cell Injury in Diabetic Kidney Disease. Front Med (Lausanne). 2021;8: 661076. [51] LECHNER C, MöNNING U, REICHEL A, et al. Potential and Limits of Kidney Cells for Evaluation of Renal Excretion. Pharmaceuticals (Basel). 2021;14(9):908. [52] YU P, ZHU H, BOSHOLM CC, et al. Precision nephrotoxicity testing using 3D in vitro models. Cell Biosci. 2023;13(1):231. [53] JENKINSON SE, CHUNG GW, VAN LOON E, et al. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch. 2012;464(6):601-611. [54] SHRESTHA S, HAQUE ME, IGHOFOSE E, et al. Primary and Immortalized Cultures of Human Proximal Tubule Cells Possess Both Progenitor and Non-Progenitor Cells That Can Impact Experimental Results. J Pers Med. 2023;13(4):613. [55] JIANG S, SU H. Cellular crosstalk of mesangial cells and tubular epithelial cells in diabetic kidney disease. Cell Commun Signal. 2023; 21(1):288. [56] CASALENA GA, YU L, GIL R, et al. The diabetic microenvironment causes mitochondrial oxidative stress in glomerular endothelial cells and pathological crosstalk with podocytes. Cell Commun Signal. 2020;18(1):105. [57] BREYER MD, BöTTINGER E, BROSIUS FC, 3RD, et al. Mouse models of diabetic nephropathy. Am Soc Nephrol. 2005;16(1):27-45. [58] XU J, HUANG Y, LI F, et al. FVB mouse genotype confers susceptibility to OVE26 diabetic albuminuria. Am J Physiol Renal Physiol. 2010; 299(3):F487-494. [59] WANG W, JIANG S, TANG X, et al. Sex differences in progression of diabetic nephropathy in OVE26 type 1 diabetic mice. Biochim Biophys Acta Mol Basis Dis. 2020;1866(1):165589.
[60] ZHENG S, HUANG Y, YANG L, et al. Uninephrectomy of diabetic OVE26 mice greatly accelerates albuminuria, fibrosis, inflammatory cell infiltration and changes in gene expression. Nephron Exp Nephrol. 2011;119(1):e21-32.
[61] NAKAGAWA T, SATO W, GLUSHAKOVA O, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. JAm Soc Nephrol. 2007;18(2):539-550. [62] SØGAARD SB, ANDERSEN SB, TAGHAVI I, et al. Super-Resolution Ultrasound Imaging of Renal Vascular Alterations in Zucker Diabetic Fatty Rats during the Development of Diabetic Kidney Disease. Diagnostics (Basel). 2023;13(20):3197. [63] HUDKINS KL, PICHAIWONG W, WIETECHA T, et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol. 2010;21(9):1533-1542. [64] YAMAMOTO Y, KATO I, DOI T, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108(2):261-268. [65] INAGI R, YAMAMOTO Y, NANGAKU M, et al. A severe diabetic nephropathy model with early development of nodule-like lesions induced by megsin overexpression in RAGE/iNOS transgenic mice. Diabetes. 2006;55(2):356-366. [66] OPAZO-RíOS L, SANCHEZ MATUS Y, RODRIGUES-DíEZ RR, et al. Anti-inflammatory, antioxidant and renoprotective effects of SOCS1 mimetic peptide in the BTBR ob/ob mouse model of type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001242. [67] THIBODEAU JF, HOLTERMAN CE, BURGER D, et al. A novel mouse model of advanced diabetic kidney disease. PloS one. 2014;9(12):e113459. [68] DU J, DONG W, LI H, et al. Protective effects of IFN-γ on the kidney of type- 2 diabetic KKAy mice. Pharmacol Rep. 2018;70(3):607-613. [69] CHANDER PN, GEALEKMAN O, BRODSKY SV, et al. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen.J Am Soc Nephrol. 2004;15(9):2391-2403. [70] JOHN A, AMIRI L, SHAFARIN J, et al. Alterations in Energy Metabolism, Mitochondrial Function and Redox Homeostasis in GK Diabetic Rat Tissues Treated with Aspirin. Life (Basel). 2022;12(1):104. [71] NOBREGA MA, FLEMING S, ROMAN RJ, et al. Initial characterization of a rat model of diabetic nephropathy. Diabetes. 2004;53(3):735-742. [72] WAUMAN J, ZABEAU L, TAVERNIER J. The Leptin Receptor Complex: Heavier Than Expected?. Front Endocrinol (Lausanne). 2017:8:30. [73] CHOW F, OZOLS E, NIKOLIC-PATERSON DJ, et al. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65(1):116-128. [74] TALUKDAR A, BASUMATARY M. Rodent models to study type 1 and type 2 diabetes induced human diabetic nephropathy. Mol Biol Rep. 2023;50(9):7759-7782. [75] SURIANO F, VIEIRA-SILVA S, FALONY G, et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: two sides of the same coin. Microbiome. 2021;9(1):147. [76] WALTER DL, THUMA JR, MALGOR R, et al. Consequences of Both Coxsackievirus B4 and Type 1 Diabetes on Female Non-Obese Diabetic Mouse Kidneys. Microorganisms. 2021;9(11):2357. [77] AZUSHIMA K, GURLEY SB, COFFMAN TM. Modelling diabetic nephropathy in mice. Nat Rev Nephrol. 2018;14(1):48-56. [78] VELASQUEZ MT, KIMMEL PL, MICHAELIS OET. Animal models of spontaneous diabetic kidney disease. FASEB J. 1990;4(11):2850-2859. [79] MELEZ KA, HARRISON LC, GILLIAM JN, et al. Diabetes is associated with autoimmunity in the New Zealand obese (NZO) mouse. Diabetes. 1980;29(10):835-840. [80] DOI T, HATTORI M, AGODOA LY, et al. Glomerular lesions in nonobese diabetic mouse: before and after the onset of hyperglycemia. Lab Invest. 1990;63(2):204-212. [81] MAEDA M, YABUKI A, SUZUKI S, et al. Renal lesions in spontaneous insulin-dependent diabetes mellitus in the nonobese diabetic mouse: acute phase of diabetes. Vet Pathol. 2003;40(2):187-195. [82] KATSUDA Y, OHTA T, MIYAJIMA K, et al. Diabetic complications in obese type 2 diabetic rat models. Exp Anim. 2014;63(2):121-132. [83] GIRALT-LÓPEZ A, MOLINA-VAN DEN BOSCH M, VERGARA A, et al. Revisiting Experimental Models of Diabetic Nephropathy. Int J Mol Sci. 2020;21(10):3587. [84] 叶桐江,郑博文,赵琳,等. 链脲佐菌素诱导1型糖尿病大鼠模型的最佳禁食时间与最优剂量[J].兰州大学学报(医学版),2019, 45(2):52-55. [85] 冷昌龙,皮明山,龚晓康.单次大剂量对比多次小剂量STZ诱导C57BL/6J小鼠糖尿病肾病模型的研究[J]. 中国比较医学杂志,2021, 31(9):113-118. [86] FURMAN BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1(4):e78. [87] 修贤杰,张萍,郑小鹏,等.多因素干预对STZ制备糖尿病肾病模型的影响研究[J].糖尿病新世界,2019,22(14):197-198. [88] 黎娅,吴穹,马晓雨,等.高脂饮食和链脲佐菌素建立2型糖尿病大鼠模型的影响因素[J].菏泽医学专科学校学报,2020,32(1):91-93. [89] 李小会,王琦,贾国华,等.STZ诱导联合高脂饮食及右肾切除糖尿病肾病模型大鼠病程早、中、晚期的界定[J].中国中西医结合肾病杂志,2019,20(8):667-670+655. [90] 闫永恒,李渐鹏,刘战伟,等.不同2型糖尿病肾病模型建立方法及成模特点比较[J].中国食物与营养,2016,22(1):65-69. [91] JIMéNEZ-CASTILLA L, MARíN-ROYO G, OREJUDO M, et al. Nephroprotective Effects of Synthetic Flavonoid Hidrosmin in Experimental Diabetic Nephropathy. Antioxidants (Basel). 2021;10(12): 1920. [92] ORABY MA, EL-YAMANY MF, SAFAR MM, et al. Amelioration of Early Markers of Diabetic Nephropathy by Linagliptin in Fructose-Streptozotocin-Induced Type 2 Diabetic Rats. Nephron. 2019;141(4):273-286. [93] 徐孝平,寿旗扬,陈方明,等.高热量高蛋白饮食诱导GK大鼠糖尿病肾病模型的建立[J].中国比较医学杂志,2012,22(4):53-57+92. [94] 印红爱,吴勇军,喻嵘,等.高脂喂养加单侧肾切除制作小鼠糖尿病肾病模型研究[J].湖南中医药大学学报,2013,33(11):17-18+27. [95] 祁珊珊,何佳,孙泽,等.糖尿病肾病SD大鼠模型的建立和评价[J].中国临床药理学杂志,2021,37(9):1114-1116+1125. [96] 文雪,马跃荣.糖尿病肾病病证结合动物模型研究进展[J].亚太传统医药,2018,14(5):117-119. [97] 黄越燕.病证结合动物模型的研究现状与思考[J].世界中西医结合杂志,2018,13(10):1459-1462. [98] 庞欣欣,彭紫凝,邢玉凤,等.基于病证结合探讨血瘀证糖尿病肾病大鼠肾损害与内质网应激的关系[J].中国实验方剂学杂志, 2020,26(20):74-81. [99] 韩佳瑞,邢玉凤,彭紫凝,等.基于病证结合探讨糖尿病肾病阴虚证大鼠肾损害与自噬的关系[J].世界科学技术-中医药现代化, 2021,23(3):938-947. [100] 高云霄,蔺亚东,彭菊琴,等.KK-Ay小鼠糖尿病肾病模型气阴两虚证的演变与评价[J].中华中医药杂志,2023,38(8):3830-3835. [101] 陈鹏德,姚蓝,郭凤,等.基于肠道菌群探讨气阴两虚证糖尿病肾脏疾病动物模型[J].天然产物研究与开发,2023,35(12):2040-2048. |
| [1] | Fang Yuan, Qian Zhiyong, He Yuanhada, Wang Haiyan, Sha Lirong, Li Xiaohe, Liu Jing, He Yachao, Zhang Kai, Temribagen. Mechanism of Mongolian medicine Echinops sphaerocephalus L. in proliferation and angiogenesis of vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7519-7528. |
| [2] | Li Chen, Liu Ye, Ni Xindi, Zhang Yuang. Simulation analysis of real-time continuous stiffness in muscle fibers and tendons of the triceps surae during multi-joint movement [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7529-7536. |
| [3] | Yang Bo, Pan Xinfang, Chang Liuhui, Ni Yong. Correlation of echocardiographic parameters with disability at 3 months after acute ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7544-7551. |
| [4] | Liu Xuan, Ding Yuqing, Xia Ruohan, Wang Xianwang, Hu Shujuan. Exercise prevention and treatment of insulin resistance: role and molecular mechanism of Keap1/nuclear factor erythroid2-related factor 2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7578-7588. |
| [5] | Gong Yuehong, Wang Mengjun, Ren Hang, Zheng Hui, Sun Jiajia, Liu Junpeng, Zhang Fei, Yang Jianhua, Hu Junping. Machine learning combined with bioinformatics screening of key genes for pulmonary fibrosis associated with cellular autophagy and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7679-7689. |
| [6] | Han Jie, Pan Chengzhen, Shang Yuzhi, Zhang Chi. Identification of immunodiagnostic biomarkers and drug screening for steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7690-7700. |
| [7] | Lu Xiuli, Xu Huazhen, Chen Yuxing, Yao Nan, Hu Zixuan, Huang Dane. Mechanism of Jiangu Formula in treating osteoporosis based on osteoclast-osteoblast coupling [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6828-6835. |
| [8] | Yan Laijun, Ge Haiya, Wang Zhengming, Yang Zongrui, Niu Lifeng, Zhan Hongsheng. Mechanism by which Tongdu Huoxue Decoction inhibits macrophage inflammation to delay intervertebral disc degeneration in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6851-6857. |
| [9] | Nigeayi · Aihemaiti, Yilidanna · Dilixiati, An Wei, Maimaitituxun · Tuerdi. Expression of mitochondrial creatine kinase 2 in a rat model of temporomandibular joint osteoarthritis and its role in inflammation progression [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6877-6884. |
| [10] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [11] |
Zhou Rulin, Hu Yuanzheng, Wang Zongqing, Zhou Guoping, Zhang Baochao, Xu Qian, Bai Fanghui.
Exploration of biomarkers for moyamoya disease and analysis of traditional Chinese medicine targets#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6927-6938.
|
| [12] | Zhao Xuemei, Wang Rui, Ao · Wuliji, Bao Shuyin, Jiang Xiaohua. Effects of Agiophyllum Oligo Saccharides on inflammation and apoptosis of mouse synovial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6939-6946. |
| [13] | Zhu Jiaping, Gao Bo, Lou Chunbiao, Yang Fengyong, Yang Kun. Monomeric traditional Chinese medicine in the treatment of rheumatoid arthritis: regulation of T cell balance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6955-6962. |
| [14] | Yao Tingfeng, Liu Lin, Liu Shixuan, Lu Xinyue. Meta-analysis of the effectiveness of dry needling at myofascial trigger points in the treatment of knee disorders [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6989-6996. |
| [15] | Wu Zhenhua, Zhang Xiwei, Wang Yipin, Li Qianqian. Relationship between seven serum lipid traits and osteoarthritis: a large sample analysis of European population in IEU OPEN GWAS database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 7004-7014. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||