Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (17): 3624-3631.doi: 10.12307/2025.189
Previous Articles Next Articles
Animal model of cervical spondylosis and its internal molecular mechanism
Qian Jiaming1, Wang Xiaole2, Fang Ting2, Zhou Maosheng2, Liu Fushui1, 2
- 1Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; 2Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang 330006, Jiangxi Province, China
-
Received:2024-02-27Accepted:2024-04-29Online:2025-06-18Published:2024-11-02 -
Contact:Liu Fushui, Professor, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang 330006, Jiangxi Province, China -
About author:Qian Jiaming, Doctoral candidate, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China -
Supported by:National Natural Science Foundation of China, No. 82360940 (to LFS); Key Project of Jiangxi Natural Science Foundation, No. 20224ACB206041 (to LFS); Jiangxi Province Graduate Innovation Special Fund Project, No. YC2023-B223 (to QJM)
CLC Number:
Cite this article
Qian Jiaming, Wang Xiaole, Fang Ting, Zhou Maosheng, Liu Fushui, . Animal model of cervical spondylosis and its internal molecular mechanism[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3624-3631.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
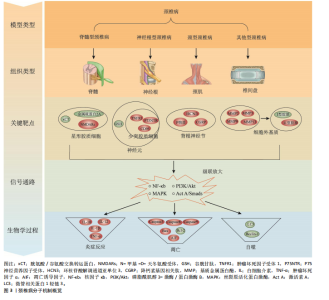
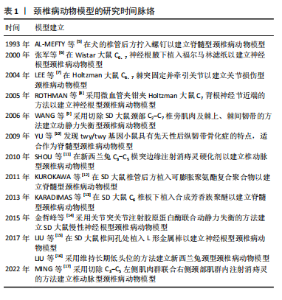
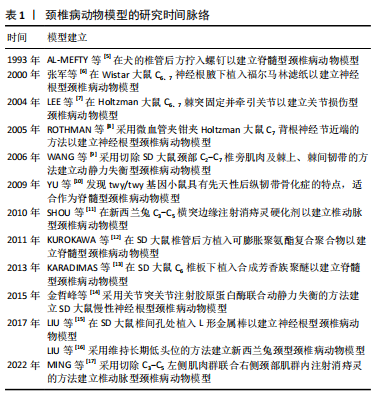
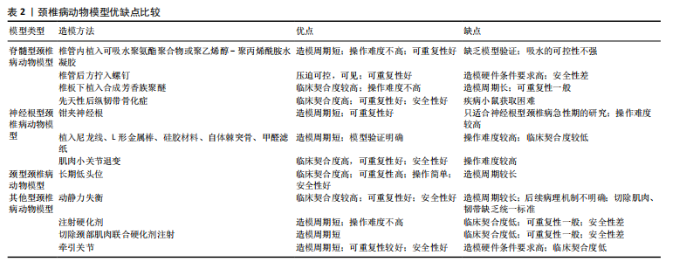
2.2 动物种类 在选择动物种类时,首要考虑的是结合人类颈椎病的发病特点,需要选择与人类解剖结构和生理功能接近、能体现颈椎退行性变的病理机制的动物种类模型。生物力学特性是选择动物模型的重要因素。在研究颈椎病的生物力学问题时,使用灵长类等大型动物是有必要的,其中恒河猴与人类的契合度最高[18],是研究人类疾病的首选。但对许多课题组来说,往往因为驯养条件严格、伦理问题和实验经费问题而无法使用。除猿、猴等动物外,羊的头部朝上,颈椎承受压力,能够很好地模拟人类生理结构,是研究颈椎病的理想动物,但因实验费用高、饲养场地要求严格,使得大样本研究受限。 从物种等级的角度考虑,在选择所用动物种类时,能用低级别的动物进行研究就不选择高等级动物。这一原则使得选择大鼠进行颈椎病造模是最多的,其次是新西兰兔,而选择羊和犬进行疾病造模的研究很少。大鼠和新西兰兔因实验成本相对较低而备受欢迎,新西兰兔体型稍大,更适合研究颈椎病等肌肉骨骼疾病,但在研究疾病分子机制时可能缺乏与人类相对应的基因或抗体,同源性问题影响实验顺利进行;此外,兔子容易生病,死亡率高,这使得大鼠成为最受欢迎的颈椎病研究动物。 值得注意的是,与人类共享95%以上基因组的小鼠,可以很容易地通过基因敲除来构建疾病模型[19]。研究发现twy/twy小鼠有先天性后纵韧带骨化症,是一种特别适合研究脊髓型颈椎病的动物模型。 2.3 动物性别 动物性别这一因素往往被一些研究人员忽视。研究发现,在颈椎病研究中雄性动物似乎更受欢迎,这可能与雄性动物受性激素影响小、行为稳定、指标检测结果稳定有关。然而,大量使用雄性实验动物容易导致实验结果因为忽略性别差异而出现偏差。缺乏雌性实验动物及对研究结果缺乏依据性别进行的分析,可能也是生物医学领域临床前研究存在可重复性问题的原因,因此性别的选择很重要。考虑到性别差异的可能性,美国国立卫生研究院的政策建议选取动物时应包含有雄性和雌性[20],在动物实验中也要“性别平等”。笔者认为在条件允许的情况下研究颈椎病最好保证所用动物雌雄各半,以排除性别差异。 2.4 动物年龄 颈椎病是一种退行性疾病,椎间盘退变在人的前20年已经发生[21],呈现年轻化、普遍化发病趋势[22],年龄越大,颈椎病的症状越严重。据报道,6月龄大鼠相当于18岁人类[23],6月龄兔子相当于16岁人类[24],小鼠性成熟年龄在8-12 周之间[25]。通过查阅大量文献发现,研究使用的大鼠年龄在6-12周之间[26-27],小鼠年龄在16-24周之间[28],兔子年龄在2-6月龄之间[29-30]。由此可见,目前研究所使用动物的年龄普遍偏小。笔者认为在选择动物模型时应将动物年龄与人类年龄进行对照,选择成年前后的动物(如大鼠和兔子选择6月龄,小鼠选择8-12周龄),造模更容易成功的同时更好地模拟了人类颈椎病的发病特点,为临床研究提供坚实的基础。 2.5 颈椎病的动物模型 根据模型的特点将颈椎病动物模型分为脊髓型、神经根型、颈型和其他型,从疾病模拟性、模型可重复性、操作简易性、造模周期、安全性等不同维度评价各种造模方法的优缺点,总结动物模型中及其干预实验中发现的分子机制,为后续研究者提出课题假说扩宽思路,具体如图3和表2。 2.5.1 脊髓型颈椎病 脊髓型颈椎病以脊髓受到压迫为特点。在椎管内植入可吸水聚氨酯聚合物或聚乙烯醇-聚丙烯酰胺水凝胶以压迫脊髓,已有研究在SD大鼠[31]、Wistar大鼠中进行[32],均未报道模型验证。笔者认为植入吸水材料的方法造模周期短,但不适合慢性脊髓压迫的研究,并且这种造模方法可控性不强;在椎管后方拧入螺钉是一种理想的造模方法,应用较多,但应循序渐进,加强观察,必要时采取措施,避免动物死亡。对于模型的验证,可以采取拍摄X射线片[33]、CT[34]、MRI或直接观察脊髓标本评估压迫程度[5,35],笔者认为需要根据实验目的来选择模型验证方法。芳香族聚醚具有促进"
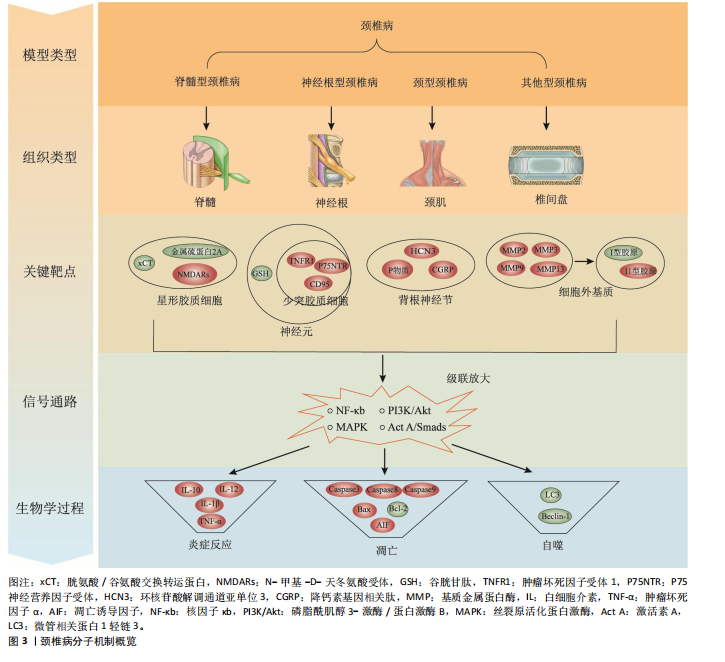

钙化的作用,有研究在SD大鼠C6椎板下植入合成芳香族聚醚[13],经MRI证实C6水平的颈脊髓后前部受压,该项研究的造模周期尚不清楚,而在新西兰兔上的研究造模周期长达20周[29],因此该方法可能存在造模周期过长的缺陷。小鼠先天性后纵韧带骨化症是一种理想的动物模型,特别适合后纵韧带骨化症的研究,可以直接根据经轴连续切片分析脊髓横向受压残余区[28],也可以间接从特征性脚趾行走模式来识别[10]。随着时间的推移,小鼠脊髓型颈椎病症状逐渐加重,这与人类的发病过程一致,使用该动物模型进行的基础研究将具有重要的临床参考价值。 在脊髓型颈椎病动物模型中,脊髓受压后Caspase-3、Caspase-8、Caspase-9[10]、凋亡诱导因子等凋亡相关分子表达升高[36],核因子κB/p50、核因子κB/p65以及基质金属蛋白酶2、基质金属蛋白酶9和尿激酶型纤溶酶原激活剂表达增加[29],twy/twy小鼠神经元和少突胶质细胞中的肿瘤坏死因子受体1、CD95和P75神经营养因子受体表达增加[28],表明以上分子均可能参与到脊髓型颈椎病中。在动物模型的干预实验中,前列腺素E1衍生物[12]、粒细胞集落刺激因子均可保护慢性脊髓受压大鼠的运动神经元[37],防止实验动物运动功能的下降;手术减压后血清素能纤维恢复,生长相关蛋白43表达增加[38]。麝香酮通过调节动力相关蛋白1依赖的线粒体裂变抑制炎症反应和神经元损伤[39]。利鲁唑通过减少星形胶质细胞中的 N-甲基-D-天冬氨酸受体磷酸化有效保护脊髓组织、保留神经功能和减轻神经性疼痛[40]。唑尼沙胺增加原代星形胶质细胞中胱氨酸/谷氨酸交换转运蛋白和金属硫蛋白2A表达,增加神经元中谷胱甘肽水平[32]。以上干预实验均为药物治疗,对模型的造模方法要求不高。 2.5.2 神经根型颈椎病 神经根型颈椎病以神经根受刺激为病理特征,将造模方法分为直接刺激和间接刺激。直接刺激是应用适当大小的力钳夹神经根,已有研究均在Holtzman大鼠上进行,钳夹时间在10-60 min之间[41-42]。钳夹神经根的方法仅为短期刺激,神经功能在2周左右基本恢复,因此特别适用于神经根型颈椎病急性期的研究。间接刺激是采用在神经根附近植入异物的方法,所使用的材料大多为尼龙线,质地较轻,对神经根有躲避性,因此很难保证尼龙线对神经根的连续压迫,使用双股尼龙线一定程度可避免这个问题。一项研究采用尼龙线压迫脊髓[43],却研究神经根型颈椎病,这值得商榷。相比于尼龙线,植入L形金属棒似乎更理想[15],可以达到持续压迫的效果。使用硅胶材料更能模拟椎间盘突出时突出物软弹、质韧的特点[44]。笔者认为使用自体棘突骨能够有效避免异物排斥的问题,亦是不错的选择。植入甲醛滤纸具有物理和化学的双重刺激,被应用较多,在神经根型颈椎病的基础研究中占有一定的地位[6]。各造模实验在模型验证上比较统一,根据神经根损伤后的感觉和运动功能障碍,采用动物行为表现进行评价,如自发疼痛和步态评分等。肌肉小关节退变是采用切除颈部肌肉和韧带造成动静力失衡的同时联合注射胶原蛋白酶造成关节突关节退变的方法[14],在颈椎X射线片、神经功能检测上均得到验证,契合度高,安全性好,值得推广。 在神经根型颈椎病动物模型中,神经受到刺激后背根神经节中环核苷酸解调通道亚单位3表达升高[15]。在神经根的横断和压迫模型对比中[8],P物质和降钙素基因相关肽存在差异,侧面反映它们在疼痛症状中的潜在作用,表明以上分子均可能参与到神经根型颈椎病中。在动物模型的干预实验中,MiR-204靶向胶质细胞源性神经营养因子,提高p-磷脂酰肌醇3-激酶/蛋白激酶B、p-胞外信号调控激酶1/2和Bcl-2的表达,对神经性疼痛起到保护作用[45]。艾灸治疗可通过增加微管相关蛋白1轻链3[46]、Beclin-1自噬相关表达[47],降低Bax凋亡相关表达,发挥镇痛作用,可能与激活素A/Smads信号通路有关[48]。干预实验为艾灸,需要选择解剖结构完整的动物模型以保证艾灸发挥作用。 2.5.3 颈型颈椎病 颈型颈椎病是颈椎病的早期阶段,在长期低头的白领、学生中高发。长期低头的造模方法可以模拟人长期维持低头姿势所导致的颈椎病,这种方法需要将动物放入特制的造模盒中[16],使其维持低头屈曲45°的体位,每天一次,每次5 h,造模时间长达3个月,特别适合颈型颈椎病的研究。有些研究采用风、寒、湿相结合的方法来模拟人类真实生活中受到的不良刺激[30],这种造模方法与人类颈椎病患病过程契合度高,但造模时间长且需要消耗实验人员较大的精力。 在颈型颈椎病动物模型的干预实验中,针刀可抑制颈后伸肌细胞和髓核细胞中Caspase-3、Bax蛋白表达,促进Bcl-2蛋白表达,抑制颈后伸肌细胞、髓核细胞凋亡,修复劳损颈肌,减缓颈椎病兔椎间盘退变[16,30]。针刀往往通过调筋治骨发挥作用,因此干预方式为针刀时需要保留颈部肌肉以保证针刀发挥作用。 2.5.4 其他型颈椎病 颈椎病的发病机制之一是动静力平衡失调,颈部肌肉控制动力平衡,关节和韧带控制静力平衡。大量研究通过切除SD大鼠颈部浅层和深层肌肉、棘上韧带、棘间韧带、棘突等造成颈椎动静力失衡,引起颈椎病[9,49];在处理肌肉断端的问题上,采取切除1.5 cm肌肉或缝线结扎的方法防止肌肉断端愈合,这种造模方法是通过破坏颈部生理结构加速颈椎病的发生,与人类颈椎病发病相似。模型验证上,使用苏木精-伊红染色或X射线片进行评估。笔者认为该造模方法存在以下缺点:造模周期较长,如何进一步缩短造模周期有待思考,而部分研究在术后7 d就开始治疗,这值得商榷[50-51];后续病理机制不明确,随着时间的推移,椎间盘退变日趋严重,可能发展成脊髓型、神经根型或其他型颈椎病;肌肉和韧带切除的具体部位尚没有形成统一的标准,难以推广应用。 在动静力失衡型颈椎病模型中,造模后炎症因子白细胞介素10、白细胞介素12、白细胞介素1β[52]、肿瘤坏死因子α水平升高,核因子κB、基质金属蛋白酶3水平升高[26]。在动物模型的干预实验中,骨碎补总黄酮通过抑制丝裂原激活蛋白激酶通路减少基质金属蛋白酶3、基质金属蛋白酶13和炎症因子白细胞介素1β、肿瘤坏死因子α的表达,从而预防椎间盘退变[53]。针刺通过可增加Ⅰ型、减少Ⅱ型胶原蛋白的生成改善椎间盘退变髓核的微观结构[27]。电针治疗通过肿瘤坏死因子α-肿瘤坏死因子受体1-Caspase-8和整合素β1/蛋白激酶B信号通路以及线粒体依赖性途径抑制纤维环细胞凋亡[50-51]。捏脊治疗可能通过影响基质金属蛋白酶/基质金属蛋白酶组织抑制因子 1的代谢平衡,进而调节细胞外基质代谢系统,达到延缓椎间盘退变的目的[54]。动静力失衡型颈椎病模型在药物、针刺、电针和捏脊等干预治疗方面均有应用,可能与造模后恢复较长时间相关功能受影响较小有关。 对于椎动脉型颈椎病,大多数造模方法是在椎动脉旁注射硬化剂,破坏椎动脉的正常结构,4周后采用经颅多普勒超声检测椎动脉血流速度,验证造模效果。这种方法造模周期短,但临床契合度差,需要注意注射部位和硬化剂的剂量,以避免动物死亡。一项研究强调硬化剂注射剂量和周期是造模成功的关键[55],注射于大鼠左侧C3-C5横突孔周围肌肉,根据大鼠体质量每100 g注射1 mL消痔灵注射液,1周后重复注射1次,剂量同前。也有一些研究采用混合造模法[17],如采取切断大鼠单侧肌肉联合颈部注射消痔灵的方法进行椎动脉型颈椎病的造模,切除颈部肌肉造成颈部偏斜,进而使椎动脉受压。 在椎动脉型颈椎病模型中,造模后α-平滑肌肌动蛋白和基质金属蛋白酶2的表达升高[11],说明颈椎横突旁注射硬化剂可诱导兔椎动脉重构,是建立椎动脉型颈椎病动物模型的一种实用方法。在动物模型的干预实验中,推拿治疗椎动脉型颈椎病兔能够改善椎动脉血流量,但需要达到20 min才能发挥作用,这种时效性特点与推拿改善兔血清中神经肽Y 和血管内皮素1 密切相关[56]。使用推拿干预这类动物模型,与临床推拿治疗椎动脉型颈椎病较为契合,可显著改善椎动脉血流量,干预效果好。 关节损伤模型是笔者根据造模方法单独归纳出来的,适合关节损伤型颈椎病的研究。造模方法是将2根钢针插入C6-C7关节突固定,利用计算机控制上下钢针移动的距离以牵拉关节囊造成关节损伤,这种方法造模时间短,不过硬件条件要求较高,目前已在Holtzman大鼠和Lamancha或Alpine山羊中运用[57-58],研究的领域集中在电生理相关的机制上,未来可拓展到分子生物学领域。"

| [1] 杨子明,李放,陈华江.颈椎病的分型、诊断及非手术治疗专家共识(2018)[J].中华外科杂志,2018,56(6):401-402. [2] RAO R. Neck pain, cervical radiculopathy, and cervical myelopathy: pathophysiology, natural history, and clinical evaluation. J Bone Joint Surg Am. 2002;84(10):1872-1881. [3] RAO RD, CURRIER BL, ALBERT TJ, et al. Degenerative cervical spondylosis: clinical syndromes, pathogenesis, and management. J Bone Joint Surg Am. 2007;89(6):1360-1378. [4] 柯尊华,王静怡.颈椎病流行病学及发病机理研究进展[J].颈腰痛杂志,2014,35(1):62-64. [5] AL-MEFTY O, HARKEY HL, MARAWI I, et al. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993;79(4):550. [6] 张军,孙树椿.神经根型颈椎病(急性期)动物模型的建立[J].中国中医骨伤科杂志,2000,8(1):14-18. [7] LEE KE, THINNES JH, GOKHIN DS, et al. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Methods. 2004;137(2): 151-159. [8] ROTHMAN SM, KREIDER RA, WINKELSTEIN BA. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine (Phila Pa 1976). 2005;30(22):2491-2496. [9] WANG YJ, SHI Q, LU WW, et al. Cervical intervertebral disc degeneration induced by unbalanced dynamic and static forces: a novel in vivo rat model. Spine (Phila Pa 1976). 2006;31(14):1532-1538. [10] YU WR, BAPTISTE DC, LIU T, et al. Molecular mechanisms of spinal cord dysfunction and cell death in the spinal hyperostotic mouse: implications for the pathophysiology of human cervical spondylotic myelopathy. Neurobiol Dis. 2009;33(2):149-163. [11] SHOU Z, SHEN L, XIONG P. Assessing the validity of a novel model of vertebral artery type of cervical syndrome induced by injecting sclerosing agent next to transverse process of cervical vertebra. J Huazhong Univ Sci Technolog Med Sci. 2010;30(1): 85-88. [12] KUROKAWA R, NAGAYAMA E, MURATA H, et al. Limaprost alfadex, a prostaglandin E1 derivative, prevents deterioration of forced exercise capability in rats with chronic compression of the spinal cord. Spine (Phila Pa 1976). 2011;36(11):865-869. [13] KARADIMAS SK, MOON ES, YU WR, et al. A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol Dis. 2013;54:43-58. [14] 金哲峰,徐凡平,朱立国,等.慢性神经根型颈椎病动物模型的建立[J].天津中医药,2015,32(9):558-562. [15] LIU DL, WANG X, CHU WG, et al. Chronic cervical radiculopathic pain is associated with increased excitability and hyperpolarization-activated current (Ih) in large-diameter dorsal root ganglion neurons. Mol Pain. 2017;13:2071456393. [16] LIU FS, ZHOU FY, ZHANG Y, et al. Effects of Acupotomy Therapy on mRNA Expressions of Bcl-2, Bax, Caspase-3 in Posterior Cervical Extensor Muscles in Cervical Spondylosis Rabbits. Zhen Ci Yan Jiu. 2017;42(6):514-517. [17] MING RR, ZHANG YQ, XU Y, et al. Mechanism of Panlongqi Tablets intervening in vertebral artery type of cervical spondylosis in rats through PI3K/AKT signaling pathway based on network pharmacology and experimental verification. Zhongguo Zhong Yao Za Zhi. 2022;47(16): 4454-4461. [18] ROGERS J. Genomic resources for rhesus macaques (Macaca mulatta). Mamm Genome. 2022;33(1):91-99. [19] XAVIER A, TOUMI H, LESPESSAILLES E. Animal Model for Glucocorticoid Induced Osteoporosis: A Systematic Review from 2011 to 2021. Int J Mol Sci. 2021;23(1):377. [20] CLAYTON JA, COLLINS FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282-283. [21] HAEFELI M, KALBERER F, SAEGESSER D, et al. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine (Phila Pa 1976). 2006;31(14):1522-1531. [22] FERNÁNDEZ-DE-LAS-PEÑAS C, ALONSO-BLANCO C, HERNÁNDEZ-BARRERA V, et al. Has the prevalence of neck pain and low back pain changed over the last 5 years? A population-based national study in Spain. Spine J. 2013;13(9):1069-1076. [23] SENGUPTA P. The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med. 2013;4(6):624-630. [24] DUTTA S, SENGUPTA P. Rabbits and men: relating their ages. J Basic Clin Physiol Pharmacol. 2018;29(5):427-435. [25] DUTTA S, SENGUPTA P. Men and mice: Relating their ages. Life Sci. 2016;152:244-248. [26] YIN J, REN K, HUANG Y, et al. Exploration about changes of IL-10, NF-κB and MMP-3 in a rat model of cervical spondylosis. Mol Immunol. 2018;93:184-188. [27] SUN Q, LIU F, GAO M, et al. Therapeutic evaluation of acupoint stimulation with needle-scapelon on rat model of degenerative cervical intervertebral discs. Biomed Pharmacother. 2019;110:677-684. [28] UCHIDA K, NAKAJIMA H, WATANABE S, et al. Apoptosis of neurons and oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy): possible pathomechanism of human cervical compressive myelopathy. Eur Spine J. 2012;21(3):490-497. [29] KARADIMAS SK, KLIRONOMOS G, PAPACHRISTOU DJ, et al. Immunohistochemical profile of NF-κB/p50, NF-κB/p65, MMP-9,MMP-2, and u-PA in experimental cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(1):4-10. [30] CHEN B, LIU H, ZHANG LZ, et al. Visual acupotomy intervention mitigates pain reaction by improving intervertebral disc degeneration and inhibiting apoptosis of nucleus pulposus cells in rabbits with cervical spondylosis. Zhen Ci Yan Jiu. 2022;47(11): 1005-1011. [31] LI GS, CHEN GH, WANG KH, et al. Neurovascular Unit Compensation from Adjacent Level May Contribute to Spontaneous Functional Recovery in Experimental Cervical Spondylotic Myelopathy. Int J Mol Sci. 2023;24(4):3408. [32] KANBARA S, OHKAWARA B, NAKASHIMA H, et al. Zonisamide ameliorates progression of cervical spondylotic myelopathy in a rat model. Sci Rep. 2020;10(1):13138. [33] XU P, GONG WM, LI Y, et al. Destructive pathological changes in the rat spinal cord due to chronic mechanical compression. Laboratory investigation. J Neurosurg Spine. 2008;8(3):279-285. [34] LEE J, SATKUNENDRARAJAH K, FEHLINGS MG. Development and characterization of a novel rat model of cervical spondylotic myelopathy: the impact of chronic cord compression on clinical, neuroanatomical, and neurophysiological outcomes. J Neurotrauma. 2012;29(5):1012-1027. [35] IBRAHIM S, ADEPUTRA NI, DANIL M, et al. Potential benefit of olive leaf extract in cervical spondylotic myelopathy model. Ann Med Surg (Lond). 2022;73:103040. [36] IBRAHIM S, MOUSA A, RIAWAN W. Expression of AIF and Caspase-3 in New Zealand rabbit with Cervical Spondylosis Myelopathy model. Ann Med Surg (Lond). 2021;69:102604. [37] YOSHIZUMI T, MURATA H, YAMAMOTO S, et al. Granulocyte Colony-Stimulating Factor Improves Motor Function in Rats Developing Compression Myelopathy. Spine (Phila Pa 1976). 2016;41(23): E1380-E1387. [38] DHILLON RS, PARKER J, SYED YA, et al. Axonal plasticity underpins the functional recovery following surgical decompression in a rat model of cervical spondylotic myelopathy. Acta Neuropathol Commun. 2016;4(1): 89. [39] ZHOU LY, YAO M, TIAN ZR, et al. Muscone suppresses inflammatory responses and neuronal damage in a rat model of cervical spondylotic myelopathy by regulating Drp1-dependent mitochondrial fission. J Neurochem. 2020;155(2):154-176. [40] MOON ES, KARADIMAS SK, YU WR, et al. Riluzole attenuates neuropathic pain and enhances functional recovery in a rodent model of cervical spondylotic myelopathy. Neurobiol Dis. 2014;62:394-406. [41] NICHOLSON KJ, ZHANG S, GILLILAND TM, et al. Riluzole effects on behavioral sensitivity and the development of axonal damage and spinal modifications that occur after painful nerve root compression. J Neurosurg Spine. 2014;20(6):751-762. [42] NICHOLSON KJ, GUARINO BB, WINKELSTEIN BA. Transient nerve root compression load and duration differentially mediate behavioral sensitivity and associated spinal astrocyte activation and mGLuR5 expression. Neuroscience. 2012;209:187-195. [43] SUN W, ZHENG K, LIU B, et al. Neuroprotective Potential of Gentongping in Rat Model of Cervical Spondylotic Radiculopathy Targeting PPAR-γPathway. J Immunol Res. 2017;2017:1-12. [44] TANG ZY, SHU B, CUI XJ, et al. Changes of cervical dorsal root ganglia induced by compression injury and decompression procedure: a novel rat model of cervical radiculoneuropathy. J Neurotrauma. 2009;26(2): 289-295. [45] SHEN WS, LI CF, ZHOU ZS, et al. MicroRNA-204 silencing relieves pain of cervical spondylotic radiculopathy by targeting GDNF. Gene Ther. 2020;27(6):254-265. [46] QIN MX, SU SY, ZHANG X, et al. Effect of mild moxibustion on pain and expression of LC3 and Bax in spinal cord in cervical spondylotic radiculopathy rats. Zhen Ci Yan Jiu. 2022;47(3):244-249. [47] LIN YY, SU SY, XU YY, et al. Effect of wheat-grain moxibustion on the expression of Beclin-1/GRP78 in spinal dorsal horn in rats with cervical spondylotic radiculopathy. Zhongguo Zhen Jiu. 2022;42(5):533-539. [48] CAI HQ, LIN XY, CHEN HY, et al. Direct moxibustion exerts an analgesic effect on cervical spondylotic radiculopathy by increasing autophagy via the Act A/Smads signaling pathway. Brain Behav. 2022;12(4):e2545. [49] XIE H, HUANG Y, NONG L, et al. X-ray dynamic observation of cervical degenerative disease induced by unbalanced dynamic and static forces in rats 1. Acta Cir Bras. 2017;32(9):736-745. [50] LIAO J, KE M, XU T, et al. Electroacupuncture inhibits apoptosis in annulus fibrosis cells through suppression of the mitochondria-dependent pathway in a rat model of cervical intervertebral disc degradation. Genet Mol Biol. 2012;35(3):686-692. [51] LIAO J, ZHANG L, ZHENG J, et al. Electroacupuncture inhibits annulus fibrosis cell apoptosis in vivo via TNF-alpha-TNFR1-caspase-8 and integrin beta1/Akt signaling pathways. J Tradit Chin Med. 2014;34(6): 684-690. [52] YIN J, HUANG Y, GAO G, et al. Changes and significance of inflammatory cytokines in a rat model of cervical spondylosis. Exp Ther Med. 2018; 15(1):400-406. [53] ZHAO K, CHEN M, LIU T, et al. Rhizoma drynariae total flavonoids inhibit the inflammatory response and matrix degeneration via MAPK pathway in a rat degenerative cervical intervertebral disc model. Biomed Pharmacother. 2021;138:111466. [54] HUANG LS, HUANG PP, TONG XB, et al. Effects of Chiropractics on Intervertebral Disk Extracell Matrix and Metabolic Enzymes in Rats with Cervical Spondylosis. Zhen Ci Yan Jiu. 2017;42(4):321-326. [55] WANG YX, LIN YK, PENG N, et al. Experimental study of improved sclerotherapy injection in rat model of vertebral arteriocervical spondylopathy. Zhongguo Gu Shang. 2023;36(2):185-188. [56] WANG C, ZHU JC, XIONG YZ, et al. Experimental study on improvement of blood supply timeliness of rabbits with vertebral artery type of cervical spondylosis by massage. Zhongguo Gu Shang. 2018;31(8): 769-774. [57] ITA ME, CROSBY ND, BULKA BA, et al. Painful Cervical Facet Joint Injury Is Accompanied by Changes in the Number of Excitatory and Inhibitory Synapses in the Superficial Dorsal Horn That Differentially Relate to Local Tissue Injury Severity. Spine (Phila Pa 1976). 2017;42(12): E695-E701. [58] KALLAKURI S, SINGH A, LU Y, et al. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17(4): 556-563. [59] 王辉昊,詹红生.颈椎病中医实验动物模型的研究进展[J].中华中医药杂志,2022,37(6):3335-3339. [60] 王诗军,李钰婷,李淳德.颈椎病动物模型的建立及其应用研究与进展[J].中国组织工程研究,2016,20(40):6067-6073. [61] 朱立国,唐彬,魏戌,等.神经根型颈椎病动物模型的研究进展及评价[J].中国组织工程研究,2018,22(35):5700-5705. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [3] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [4] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [5] | Zhao Xiaoxuan, Liu Shuaiyi, Li Qi, Xing Zheng, Li Qingwen, Chu Xiaolei. Different exercise modalities promote functional recovery after peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1248-1256. |
| [6] | Wang Rongrong, Huang Yushan, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 regulates vascular-related factors and protects microcirculatory function during the acute phase of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 958-967. |
| [7] | Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977. |
| [8] | Yang Bin, Tao Guangyi, Yang Shun, Xu Junjie, Huang Junqing . Visualization analysis of research hotspots of artificial intelligence in field of spinal cord nerve injury and repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 761-770. |
| [9] | Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838. |
| [10] | Zheng Yitong, Wang Yongxin, Liu Wen, Amujite, Qin Hu. Action mechanism of intrathecal transplantation of human umbilical cord mesenchymal stem cell-derived exosomes for repair of spinal cord injury under neuroendoscopy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7743-7751. |
| [11] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [12] | Xu Biao, Dong Yuzhen, Lu Tan. Effect of dihydroquercetin on the expression of inflammatory response markers in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6843-6850. |
| [13] | Luo Dan, Ge Zhilin, Hou Yonghui, Wang Wanshun, Zhan Jiheng, Hou Yu, Lin Dingkun, Chen Shudong. Extraction and subculture of neural stem cells from mouse embryonic spinal cord: comparison and analysis on advantages and disadvantages of three commonly used digestive enzymes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6609-6615. |
| [14] | Zhang Xuesan, Zhang Zheng, Shen Le, Geng Qingqing, Tan Shusen, Lou Chunbiao, Han Kang. Natural products modulate pyroptosis for treatment of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6520-6528. |
| [15] | Xu Zhenhua, Li Yanjie, Qin Hewei, Liu Haoyuan, Zhu Bochao, Wang Yupu. Traditional Chinese medicine monomer in treatment of neuroinflammation after spinal cord injury: effects of nuclear transcription factor kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 590-598. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||