Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (23): 4859-4867.doi: 10.12307/2025.083
Previous Articles Next Articles
Inhibitory effect of angiotensin II on the brown fat differentiation of rat bone marrow mesenchymal stem cells
Liu Chenyang, Wang Jin, Zhang Wenting, Wang Liqing, Yin Xiaoxiao, Zhao Junnan, Jiao Xiangying
- Department of Physiology, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2024-02-29Accepted:2024-05-21Online:2025-08-18Published:2024-09-26 -
Contact:Jiao Xiangying, PhD, Professor, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Liu Chenyang, Master candidate, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
CLC Number:
Cite this article
Liu Chenyang, Wang Jin, Zhang Wenting, Wang Liqing, Yin Xiaoxiao, Zhao Junnan, Jiao Xiangying. Inhibitory effect of angiotensin II on the brown fat differentiation of rat bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4859-4867.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
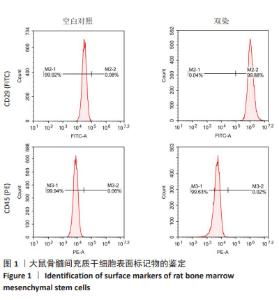
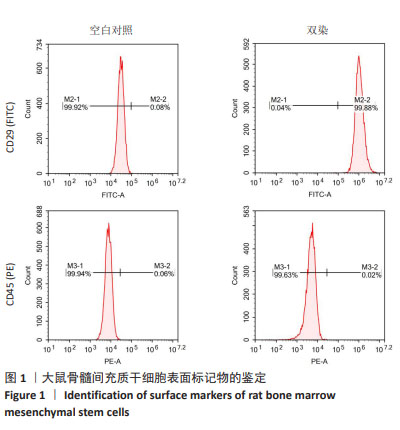
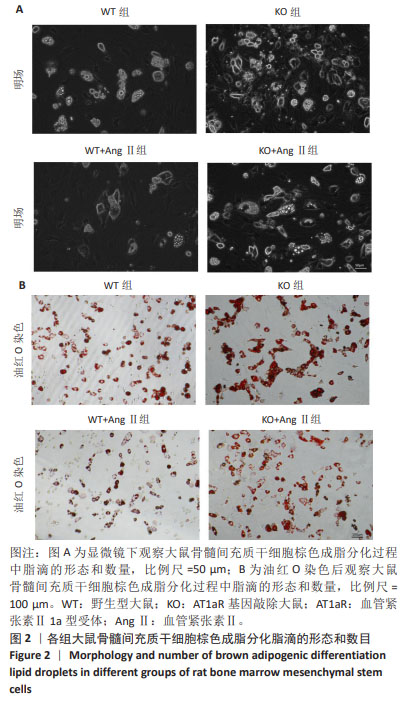
2.2 AT1aR敲除改善Ang Ⅱ对大鼠骨髓间充质干细胞棕色脂变过程中脂滴形成的抑制作用 诱导分化14 d后,在明场下观察到:与WT组相比,WT+Ang Ⅱ组大鼠骨髓间充质干细胞的脂滴数目明显减少,而敲除AT1aR后脂滴较多且呈多房状的小脂滴。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组脂滴数目明显增多,见图2A。用油红O染料进行染色,如图2B所示,与WT组相比,WT+Ang Ⅱ组脂滴着色明显减少,而敲除AT1aR后脂滴明显增多,可看到成簇的多房脂滴聚集成团。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组的脂滴着色明显增多。因此,Ang Ⅱ减少骨髓间充质干细胞棕色成脂诱导分化脂滴的形成,而AT1aR敲除可以促进骨髓间充质干细胞向棕色脂肪细胞分化过程中的脂滴形成。"
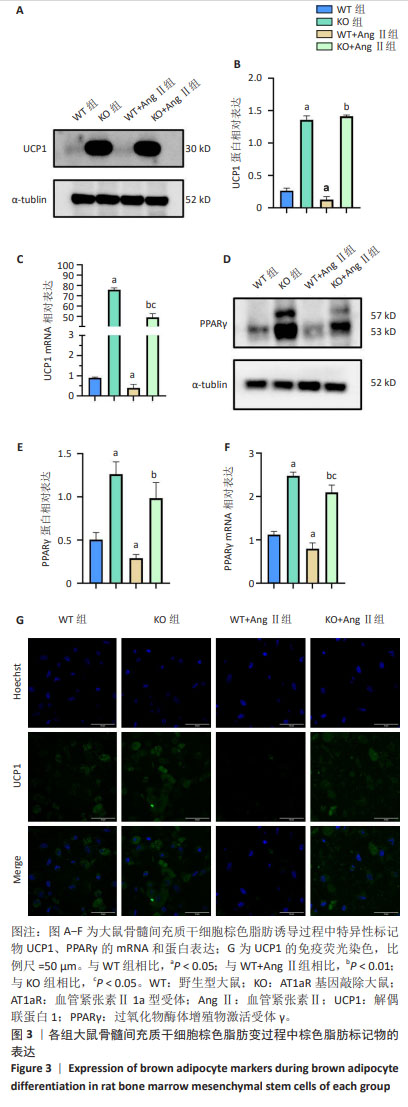
2.3 AT1aR敲除改善了AngⅡ对大鼠骨髓间充质干细胞棕色成脂的抑制作用 棕色脂肪组织最显著的特点是特异性表达UCP1,使氧化磷酸化解偶联不生成ATP,但质子梯度储存的能量以热能形式释放,使组织产热增加[8-9]。PPARγ是一种转录因子,属于核受体超家族的一员,主要参与调控脂肪细胞的分化、脂质代谢和能量平衡[10]。如图3所示,与WT组相比,WT+Ang Ⅱ组棕色脂肪变的特异性标记产物UCP1、PPARγ的mRNA及蛋白表达水平显著降低(P < 0.05),敲除AT1aR后,UCP1、PPARγ的mRNA及蛋白表达水平明显升高(P < 0.05);而与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组UCP1、PPARγ的mRNA及蛋白水平也明显升高(P < 0.01)。共聚焦显微镜观察显示,与WT组相比,WT+Ang Ⅱ组UCP1荧光强度明显减弱,UCP1表达水平降低,AT1aR敲除组UCP1荧光强度明显增强,UCP1表达水平升高。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组UCP1荧光强度也增强,UCP1表达水平升高。"
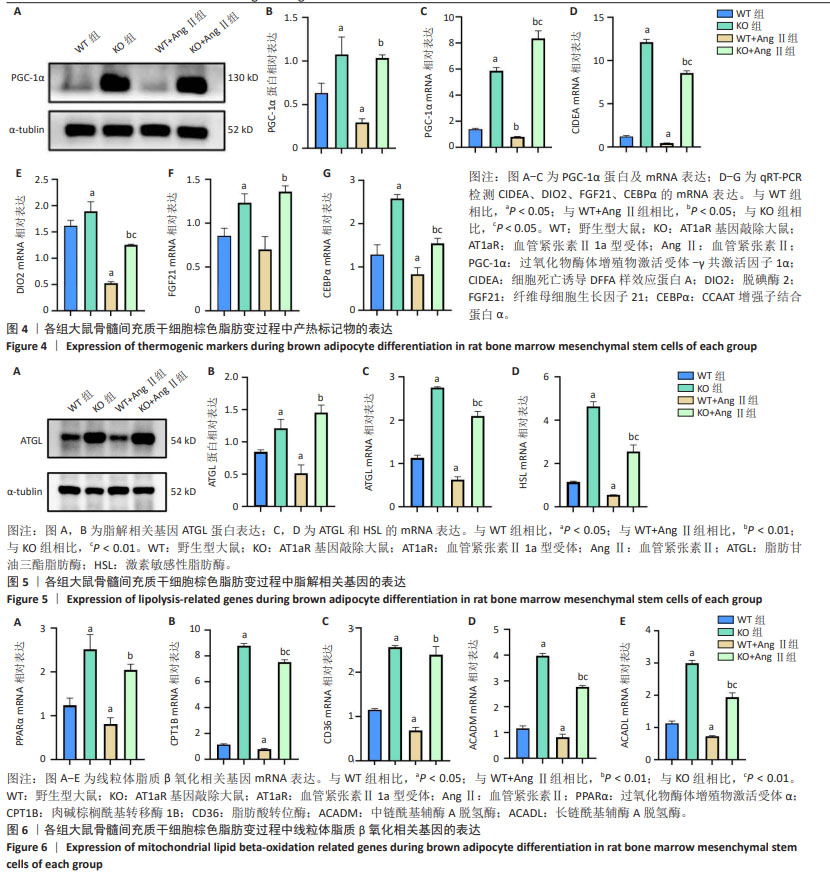
2.4 AT1aR敲除改善了AngⅡ对大鼠骨髓间充质干细胞棕色脂变过程中产热标记物表达的抑制作用 产热在改善代谢率及葡萄糖和脂质代谢中发挥着重要作用[11]。除了上述棕色脂肪特异性标记蛋白UCP1和PPARγ外,PGC-1α是一种重要的转录共激活因子,被认为在能量代谢调控中发挥着关键作用[10]。PGC-1α也是棕色脂肪细胞中产热基因表达、线粒体生物发生和氧化代谢的主要转录调控因子[12]。FGF21作为一种转录因子和激素,是维持局部和全身脂质、葡萄糖和能量代谢稳态的主要调节因子,并且被认为是一种褐变剂[13]。DIO2是一种碘代谢酶,在棕色脂肪中促进甲状腺素的转化,从而增强脂肪细胞的产热能力[14]。CIDEA最初因与凋亡DNA分解因子序列的相似性而被识别出来,且其在棕色脂肪组织中表达水平很高[15]。如图4所示,与WT组相比,WT+Ang Ⅱ组大鼠骨髓间充质干细胞棕色脂肪变过程中产热标记物PGC-1α、CEBPα、DIO2、CIDEA的mRNA表达水平显著降低(P < 0.05),AT1aR敲除组PGC-1α、CEBPα、DIO2、CIDEA、FGF21的mRNA表达水平均显著升高(P < 0.05)。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组的这些指标有明显升高(P < 0.05)。同时,PGC-1α的蛋白表达水平与qRT-PCR结果一致。以上结果表明,AT1aR敲除通过增加产热标记物的表达,进而改善Ang Ⅱ对大鼠骨髓间充质干细胞棕色脂变产热功能的不良影响。 2.5 AT1aR敲除改善了AngⅡ对大鼠骨髓间充质干细胞向棕色脂肪分化过程中脂解的抑制作用 棕色脂肪组织增多,导致机体的产热增加,因而脂肪的分解和利用增多。如图5所示,与WT组相比,WT+AngⅡ组脂肪分解限速酶ATGL和HSL的mRNA表达水平显著降低(P < 0.05),而AT1aR敲除组ATGL和HSL的mRNA表达水平显著升高(P < 0.05)。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组的这些指标有明显升高(P < 0.01)。与WT组相比,WT+Ang Ⅱ组ATGL的蛋白表达水平显著下降(P < 0.05),而AT1aR敲除组ATGL的蛋白表达水平显著升高(P < 0.05)。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组ATGL的蛋白表达水平显著升高(P < 0.01)。 2.6 AT1aR敲除改善了Ang Ⅱ对大鼠骨髓间充质干细胞脂肪酸β氧化的抑制作用 脂肪酸氧化相关酶包括ACADM、ACADL以及负责上游调节的PPAR家族[16]。有文献报道,促进游离脂肪酸代谢相关的脂肪酸β氧化速率是通过提高PPARα蛋白及其下游脂肪酸转位酶和肉碱棕榈酰基转移酶1蛋白的活性来发挥作用的[17]。在脂肪细胞中,CD36的主要作用是促进脂肪酸的摄取,它能够结合游离脂肪酸,使其进入细胞内,进而参与脂肪酸代谢[18]。如图6所示,与WT组相比,WT+Ang Ⅱ组PPARα、CPT1B、CD36、ACADM、ACADL的mRNA水平明显降低(P < 0.05),AT1aR敲除组这些指标显著升高(P < 0.05)。同时,与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组的这些指标明显升高(P < 0.01)。"
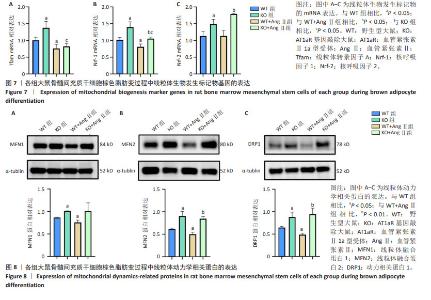
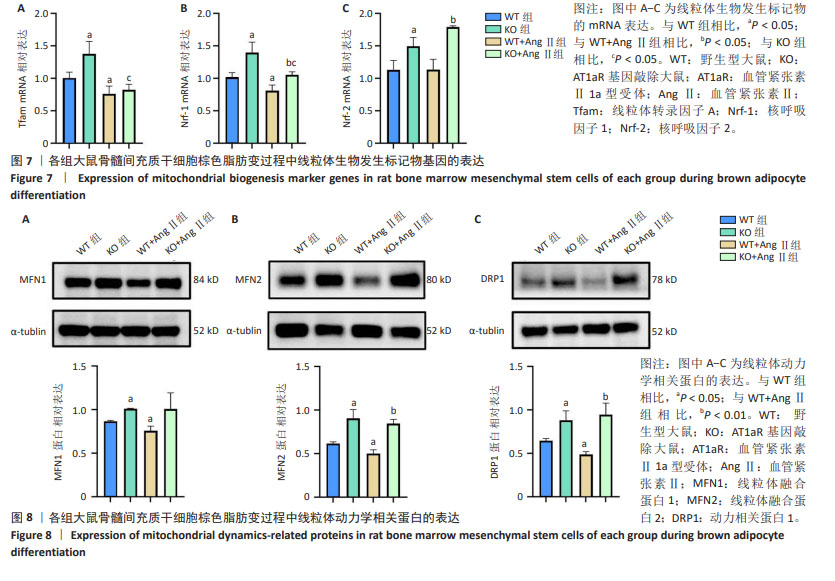
2.7 AT1aR敲除改善了AngⅡ对大鼠骨髓间充质干细胞线粒体生物发生的抑制作用 线粒体β氧化对产热至关重要,是线粒体生物发生的能量来源[10]。Tfam在调节线粒体DNA的转录和复制中发挥着关键作用[19]。Nrf-1和Nrf-2是细胞内线粒体数量和功能调节相关的转录因子,它们促进线粒体基因的表达,参与调节线粒体的生物合成和功能[20]。如图7所示,与WT组相比,WT+Ang Ⅱ组Tfam、Nrf-1的mRNA水平显著降低(P < 0.05),Nrf-2的mRNA水平无统计学差异,但是AT1aR敲除组Tfam、Nrf-1、Nrf-2的mRNA水平均显著上升(P < 0.05)。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组的Nrf-1、Nrf-2的mRNA水平也明显升高(P < 0.05),Tfam的mRNA水平虽无统计学意义,但有增高的趋势。 2.8 AT1aR敲除改善了Ang Ⅱ对骨髓间充质干细胞棕色脂变过程中线粒体功能的抑制作用 线粒体动力学在营养利用和能量消耗上有潜在作用[21]。线粒体作为一个高度动态的细胞器,不断进行融合与分裂,保持动态平衡,该过程主要由介导线粒体融合的MFN1、MFN2和介导裂变的DRP1调控[22]。如图8所示,与WT组比较,WT+AngⅡ组MFN1、MFN2、DRP1的蛋白表达水平明显降低(P < 0.05),而AT1aR敲除后MFN1、MFN2和DRP1蛋白表达水平明显升高(P < 0.05)。与WT+Ang Ⅱ组相比,KO+Ang Ⅱ组MFN2和DRP1蛋白表达水平明显升高(P < 0.01)。"
| [1] NOVELLI G, CASSADONTE C, SBRACCIA P, et al. Genetics: A Starting Point for the Prevention and the Treatment of Obesity. Nutrients. 2023;15(12):2782. [2] MATSUSHITA K, WU Y, PRATT RE, et al. Deletion of angiotensin II type 2 receptor accelerates adipogenesis in murine mesenchymal stem cells via Wnt10b/beta-catenin signaling. Lab Invest. 2016;96(8):909-917. [3] PAHLAVANI M, KALUPAHANA NS, RAMALINGAM L, et al. Regulation and Functions of the Renin-Angiotensin System in White and Brown Adipose Tissue. Compr Physiol. 2017;7(4):1137-1150. [4] CAI Z, FANG L, JIANG Y, et al. Angiotensin II Promotes White Adipose Tissue Browning and Lipolysis in Mice. Oxid Med Cell Longev. 2022;2022:6022601. [5] LI A, SHI W, WANG J, et al. The gene knockout of angiotensin II type 1a receptor improves high-fat diet-induced obesity in rat via promoting adipose lipolysis. PLoS One. 2022;17(7):e0267331. [6] LIU C, FAN Y, ZHOU L, et al. Pretreatment of mesenchymal stem cells with angiotensin II enhances paracrine effects, angiogenesis, gap junction formation and therapeutic efficacy for myocardial infarction. Int J Cardiol. 2015;188:22-32. [7] JIANG X, WU F, XU Y, et al. A novel role of angiotensin II in epidermal cell lineage determination: Angiotensin II promotes the differentiation of mesenchymal stem cells into keratinocytes through the p38 MAPK, JNK and JAK2 signalling pathways. Exp Dermatol. 2019;28(1):59-65. [8] DI MAIO G, ALESSIO N, PELUSO G, et al. Molecular and Physiological Effects of Browning Agents on White Adipocytes from Bone Marrow Mesenchymal Stromal Cells. Int J Mol Sci. 2022;23(20):12151. [9] RODRÍGUEZ-CANO MM, GONZÁLEZ-GÓMEZ MJ, SÁNCHEZ-SOLANA B, et al. NOTCH Receptors and DLK Proteins Enhance Brown Adipogenesis in Mesenchymal C3H10T1/2 Cells. Cells. 2020;9(9):2032. [10] VAN NGUYEN TT, VU VV, PHAM PV. Transcriptional Factors of Thermogenic Adipocyte Development and Generation of Brown and Beige Adipocytes From Stem Cells. Stem Cell Rev Rep. 2020;16(5):876-892. [11] KAJIMURA S, SAITO M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014;76:225-249. [12] HUANG PI, CHEN YC, CHEN LH, et al. PGC-1α mediates differentiation of mesenchymal stem cells to brown adipose cells. J Atheroscler Thromb. 2011;18(11):966-980. [13] LUO Y, YE S, CHEN X, et al. Rush to the fire: FGF21 extinguishes metabolic stress, metaflammation and tissue damage. Cytokine Growth Factor Rev. 2017;38:59-65. [14] YAU WW, SINGH BK, LESMANA R, et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy. 2019;15(1):131-150. [15] LIN SC, LI P. CIDE-A, a novel link between brown adipose tissue and obesity. Trends Mol Med. 2004;10(9):434-439. [16] WANG J, LI D, ZHANG Y, et al. Angiotensin II type 1a receptor knockout ameliorates high-fat diet-induced cardiac dysfunction by regulating glucose and lipid metabolism. Acta Biochim Biophys Sin (Shanghai). 2023; 55(9):1380-1392. [17] CHENG L, ZHANG S, SHANG F, et al. Emodin Improves Glucose and Lipid Metabolism Disorders in Obese Mice via Activating Brown Adipose Tissue and Inducing Browning of White Adipose Tissue. Front Endocrinol (Lausanne). 2021;12:618037. [18] ABE I, OGURI Y, VERKERKE ARP, et al. Lipolysis-derived linoleic acid drives beige fat progenitor cell proliferation. Dev Cell. 2022;57(23):2623-2637.e8. [19] LU T, ZHANG Z, BI Z, et al. TFAM deficiency in dendritic cells leads to mitochondrial dysfunction and enhanced antitumor immunity through cGAS-STING pathway. J Immunother Cancer. 2023;11(3):e005430. [20] CHEN QM. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic Biol Med. 2022;179:133-143. [21] WIKSTROM JD, MAHDAVIANI K, LIESA M, et al. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 2014;33(5):418-436. [22] KLEELE T, REY T, WINTER J, et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021;593(7859):435-439. [23] MARQUEZ-CURTIS LA, JANOWSKA-WIECZOREK A, MCGANN LE, et al. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology. 2015;71(2):181-197. [24] MOON H, CHOI JW, SONG BW, et al. Isoliquiritigenin Enhances the Beige Adipocyte Potential of Adipose-Derived Stem Cells by JNK Inhibition. Molecules. 2020;25(23):5660. [25] CARPENTIER AC, BLONDIN DP, HAMAN F, et al. Brown Adipose Tissue-A Translational Perspective. Endocr Rev. 2023;44(2):143-192. [26] IKEDA K, MARETICH P, KAJIMURA S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol Metab. 2018;29(3):191-200. [27] ALVAREZ-GALLEGO F, GONZÁLEZ-BLÁZQUEZ R, GIL-ORTEGA M, et al. Angiotensin II type 2 receptor as a novel activator of brown adipose tissue in obesity. Biofactors. 2023;49(6):1106-1120. [28] LOH RKC, FORMOSA MF, LA GERCHE A, et al. Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes Obes Metab. 2019;21(2):276-284. [29] SLAMKOVA M, ZORAD S, KRSKOVA K. Alternative renin-angiotensin system pathways in adipose tissue and their role in the pathogenesis of obesity. Endocr Regul. 2016;50(4):229-240. [30] NING K, LIU S, YANG B, et al. Update on the effects of energy metabolism in bone marrow mesenchymal stem cells differentiation. Mol Metab. 2022;58:101450. [31] HALLBERG M, MORGANSTEIN DL, KISKINIS E, et al. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Mol Cell Biol. 2008;28(22):6785-6795. [32] FARMER SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4(4):263-273. [33] XU Y, YU T, MA G, et al. Berberine modulates deacetylation of PPARγ to promote adipose tissue remodeling and thermogenesis via AMPK/SIRT1 pathway. Int J Biol Sci. 2021;17(12):3173-3187. [34] 常华杰,勾文峰,郭江红,等.胡黄连苷Ⅰ、Ⅱ通过调节C/EBP-PPARγ通路抑制3T3-L1脂肪前体细胞的分化和脂肪合成[J].药物评价研究, 2023,46(6):1193-1200. [35] PARK M, BAEK H, HAN JY, et al. Stevioside Enhances the Anti-Adipogenic Effect and β-Oxidation by Activating AMPK in 3T3-L1 Cells and Epididymal Adipose Tissues of db/db Mice. Cells. 2022;11(7):1076. [36] FINCK BN, KELLY DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115(19):2540-2548. [37] DUTCHAK PA, KATAFUCHI T, BOOKOUT AL, et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148(3):556-567. [38] JASH S, BANERJEE S, LEE MJ, et al. CIDEA Transcriptionally Regulates UCP1 for Britening and Thermogenesis in Human Fat Cells. iScience. 2019;20: 73-89. [39] ROSENWALD M, PERDIKARI A, RÜLICKE T, et al. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15(6):659-667. [40] YOON D, IMRAN KM, KIM YS. Distinctive effects of licarin A on lipolysis mediated by PKA and on formation of brown adipocytes from C3H10T1/2 mesenchymal stem cells. Toxicol Appl Pharmacol. 2018;340:9-20. [41] STEC DE, GORDON DM, HIPP JA, et al. Loss of hepatic PPARα promotes inflammation and serum hyperlipidemia in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2019;317(5):R733-R745. [42] PATEL BV, YAO F, HOWENSTINE A, et al. Emergent Coordination of the CHKB and CPT1B Genes in Eutherian Mammals: Implications for the Origin of Brown Adipose Tissue. J Mol Biol. 2020;432(23):6127-6145. [43] WU L, LIU C, CHANG DY, et al. The Attenuation of Diabetic Nephropathy by Annexin A1 via Regulation of Lipid Metabolism Through the AMPK/PPARα/CPT1b Pathway. Diabetes. 2021;70(10):2192-2203. [44] TAHRI-JOUTEY M, ANDREOLETTI P, SURAPUREDDI S, et al. Mechanisms Mediating the Regulation of Peroxisomal Fatty Acid Beta-Oxidation by PPARα. Int J Mol Sci. 2021;22(16):8969. [45] ZHU Q, AN YA, SCHERER PE. Mitochondrial regulation and white adipose tissue homeostasis. Trends Cell Biol. 2022;32(4):351-364. [46] ROH HC, KUMARI M, TALEB S, et al. Adipocytes fail to maintain cellular identity during obesity due to reduced PPARγ activity and elevated TGFβ-SMAD signaling. Mol Metab. 2020;42:101086. [47] CEDIKOVA M, KRIPNEROVÁ M, DVORAKOVA J, et al. Mitochondria in White, Brown, and Beige Adipocytes. Stem Cells Int. 2016;2016:6067349. [48] KLINGENBERG M, HUANG SG. Structure and function of the uncoupling protein from brown adipose tissue. Biochim Biophys Acta. 1999;1415(2): 271-296. [49] REN Z, ZHANG X, DING T, et al. Mitochondrial Dynamics Imbalance: A Strategy for Promoting Viral Infection. Front Microbiol. 2020;11:1992. [50] UM JH, YUN J. Emerging role of mitophagy in human diseases and physiology. BMB Rep. 2017;50(6):299-307. [51] TWIG G, HYDE B, SHIRIHAI OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777(9):1092-1097. [52] LIESA M, SHIRIHAI OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491-506. |
| [1] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [2] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [3] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [4] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [5] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [6] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [7] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [8] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [9] | Ge Xiao, Zhao Zhuangzhuang, Guo Shuyu, Xu Rongyao. HOXA10 gene-modified bone marrow mesenchymal stem cells promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7701-7708. |
| [10] | Zhang Xiongjinfu, Chen Yida, Cheng Xinyi, Liu Daihui, Shi Qin . Exosomes derived from bone marrow mesenchymal stem cells of young rats to reverse senescence in aged rat bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7709-7718. |
| [11] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [12] | Liu Chengyuan, Guo Qianping. Differential effects of kartogenin on chondrogenic and osteogenic differentiation of rat and rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7490-7498. |
| [13] | Cai Zhixing, Xia Qiufang, Chen Lili, Zhu Danyang, Zhu Huiwen, Sun Yanan, Liang Wenyu, Zhao Heqian. Effect of Roujishuncuiyin on the improvement of skeletal muscle insulin resistance in a mouse model of type 2 diabetes mellitus [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7537-7543. |
| [14] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [15] | Nigeayi · Aihemaiti, Yilidanna · Dilixiati, An Wei, Maimaitituxun · Tuerdi. Expression of mitochondrial creatine kinase 2 in a rat model of temporomandibular joint osteoarthritis and its role in inflammation progression [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6877-6884. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||