Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (5): 1026-1035.doi: 10.12307/2025.208
Previous Articles Next Articles
Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway
Wang Yuru1, Li Siyuan2, Xu Ye2, Zhang Yumeng2, Liu Yang2, Hao Huiqin1, 2
- 1Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan 030032, Shanxi Province, China; 2Basic Laboratory of Integrated Chinese and Western Medicine, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China
-
Received:2023-12-13Accepted:2024-01-31Online:2025-02-18Published:2024-06-04 -
Contact:Hao Huiqin, PhD, Professor, PhD supervisor, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan 030032, Shanxi Province, China; Basic Laboratory of Integrated Chinese and Western Medicine, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China -
About author:Wang Yuru, Master candidate, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan 030032, Shanxi Province, China -
Supported by:Shanxi Province Scientific Innovation Talents Team for Prevention and Treatment of Rheumatic Autoimmune Disease with Traditional Chinese and Western Medicine, No. 202204051002033 (to HHQ); Central Government Guided Local Science and Technology Development Fund Project, No. YDZJSX2021A040 (to HHQ); Construction Project of Integrated Traditional Chinese and Western Medicine Basic Discipline of Rheumatic Immune Diseases in Shanxi University of Chinese Medicine, No. 2023XKJS-03 (to HHQ)
CLC Number:
Cite this article
Wang Yuru, Li Siyuan, Xu Ye, Zhang Yumeng, Liu Yang, Hao Huiqin. Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1026-1035.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
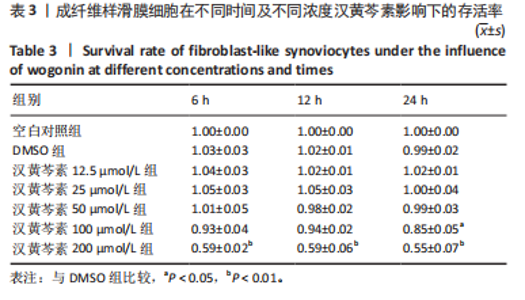
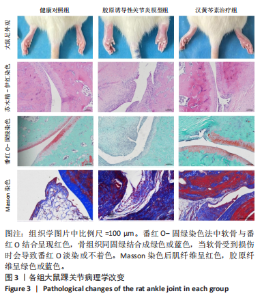
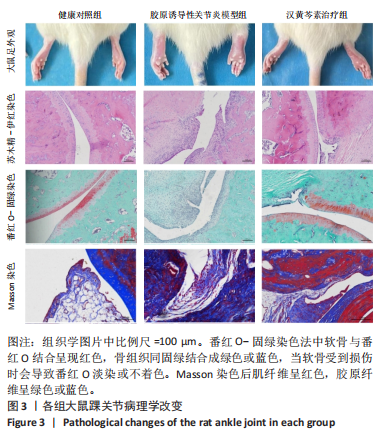
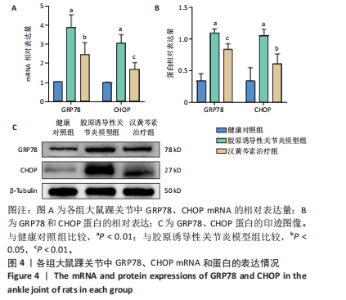
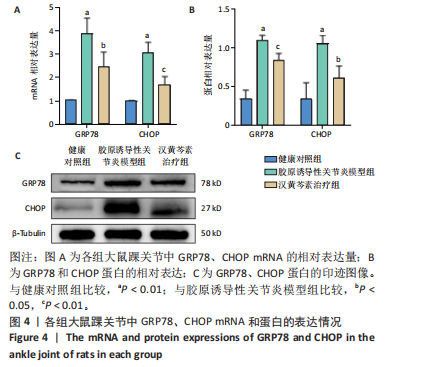
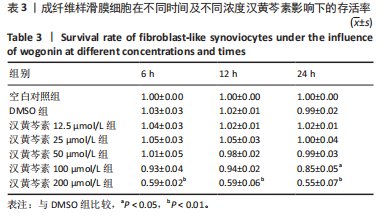
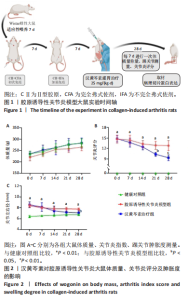
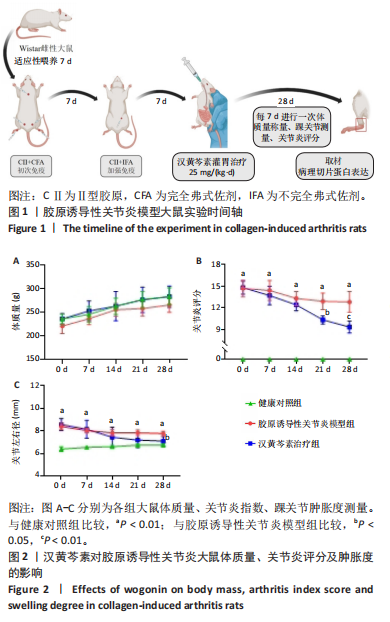
2.1 实验动物数量分析 18只大鼠实验过程中无死亡脱失,全部进入结果分析。 2.2 汉黄芩素对CIA大鼠体质量、关节肿胀度、关节炎指数评分的影响 随着饲养周期的延长,各组大鼠体质量呈现平缓增长趋势,未表现出明显差异,见图2A。足爪肿胀度和关节炎评分被认为是反映药物对CIA大鼠缓解作用最直观的指标。如图2B所示,治疗0 d时,与健康对照组相比,其余两组大鼠的关节炎指数评分均显著增高(P < 0.01),表明造模成功;到给药第21天,汉黄芩素治疗组大鼠的关节炎指数评分(10.3±0.3)较CIA模型组(12.9±0.5)明显降低(P < 0.05);治疗第28天,与CIA模型组关节炎指数评分(12.6±0.5)比较,汉黄芩素治疗组的大鼠关节炎评分(9.3±0.4)显著下降(P < 0.01),且其踝关节左右径长(7.1±0.2) mm较CIA模型组(7.8±0.1) mm显著减小(P < 0.05),见图2C。 2.3 汉黄芩素对CIA大鼠踝关节病理损伤的影响 如图3所示,给药28 d后大鼠足外观结果:CIA模型组大鼠踝关节有明显肿胀,汉黄芩素治疗组较CIA模型组肿胀程度明显减轻。病理学苏木精-伊红染色结果显示,健康对照组大鼠踝关节组织结构清晰可见,关节面光滑平整;CIA模型组大鼠踝关节结构被破坏,关节面粗糙,软骨破坏和骨质侵蚀严重,关节面及骨质内大量炎症细胞浸润;汉黄芩素治疗组病理损伤较CIA模型组明显改善,炎症细胞浸润减少,软骨破坏和骨质侵蚀出现不同程度减轻,关节结构基本清晰完整。 番红O-固绿染色结果可见健康对照组大鼠软骨完整连续,表面平整,番红O着色较深;CIA模型组几乎不可见番红O着色,关节面粗糙糜烂,可见溃疡缺损;汉黄芩素治疗组可见软骨番红O着色,关节软骨表面轻微磨损、大致光整。滑膜组织中含有较多胶原纤维,Masson染色结果示健康对照组大鼠滑膜形态正常,与关节面保持一定间距;CIA模型组关节腔内胶原染色加重,滑膜细胞增生排列紊乱并侵入关节面,关节完整结构破坏;汉黄芩素治疗组关节腔隙内有胶原增生侵袭关节面,但骨和软骨的破坏表现较CIA组有所减轻。 2.4 汉黄芩素对CIA大鼠关节GRP78、CHOP mRNA和蛋白表达的影响 qRT-PCR结果显示,与健康对照组比较,CIA模型组大鼠关节组织GRP78、CHOP mRNA相对表达量均显著升高(P < 0.01);与CIA模型组比较,汉黄芩素治疗组GRP78、CHOP mRNA相对表达量明显降低(P < 0.05,P < 0.01),见图4A。蛋白印迹检测结果示与健康对照组比较,CIA模型组大鼠关节组织GRP78、CHOP蛋白表达显著升高(均P < 0.01);与CIA模型组比较,汉黄芩素治疗组GRP78、CHOP蛋白表达明显降低(P < 0.01,P < 0.05),见图4B,C。 2.5 汉黄芩素对CIA大鼠关节GRP78、CHOP 蛋白表达的免疫组化分析 免疫组化染色结果显示,健康对照组大鼠关节中GRP78、CHOP表达很少,几乎不可见,见图5A。与健康对照组相比,CIA模型组大鼠关节中表达显著增高(P < 0.01),主要定位于侵袭的滑膜组织和被侵蚀的关节面周围;汉黄芩素治疗组相比于CIA模型组,侵入关节的滑膜组织中和关节面周围的GRP78、CHOP表达显著降低(P < 0.01),见图5B。 2.6 汉黄芩素对FLS细胞毒性的影响 不同浓度汉黄芩素分别作用FLS细胞6,12,24 h后,CCK-8实验结果见表3,DMSO组与空白对照组相比差异无显著性意义;与DMSO组相比,汉黄芩素浓度在50 μmol/L及以下时,细胞存活率未有明显下降;在100 μmol/L时,细胞存活率略有下降,但只有作用时间为24 h时差异有显著性意义(P < 0.05);在200 μmol/L时,细胞存活率均明显下降 "
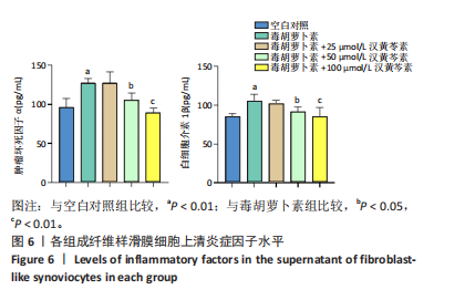
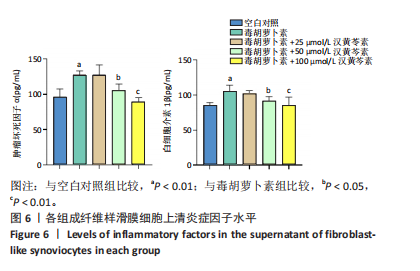
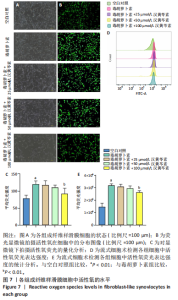
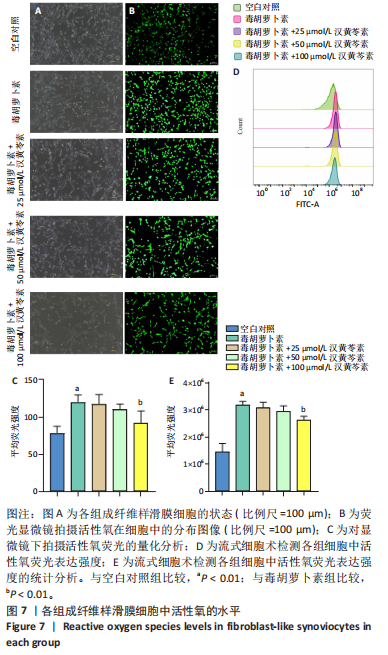
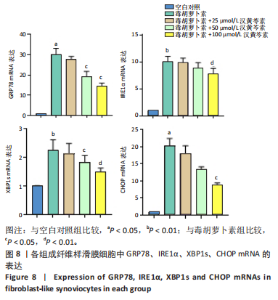
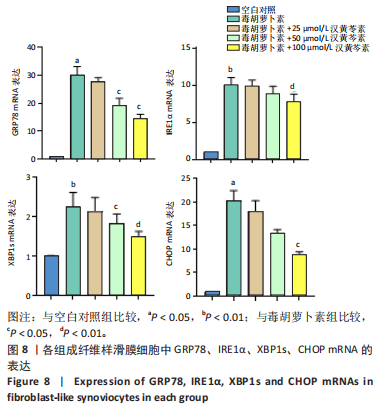
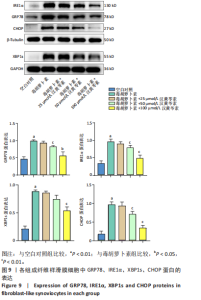
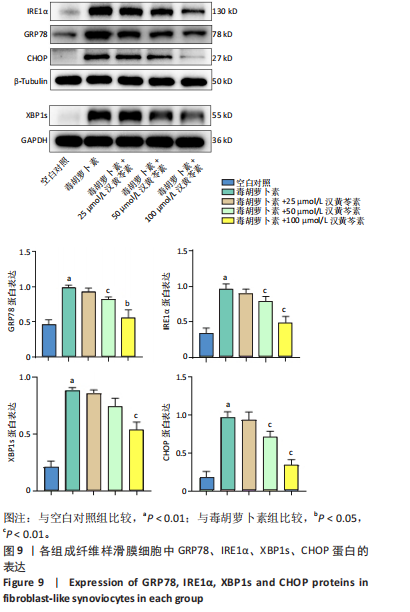
(P < 0.01)。因此,认为汉黄芩素在100 μmol/L以下时对细胞无明显的细胞毒性作用,选择25,50,100 μmol/L为后续实验汉黄芩素所采用的浓度。 2.7 各组FLS细胞上清炎症因子水平检测结果 与空白对照组相比,毒胡萝卜素组细胞上清肿瘤坏死因子α、白细胞介素1β水平显著增高(P < 0.01);与毒胡萝卜素组相比,毒胡萝卜素+25 μmol/L汉黄芩素组细胞上清肿瘤坏死因子α、白细胞介素1β无显著变化,毒胡萝卜素+50 μmol/L汉黄芩素组和毒胡萝卜素+100 μmol/L汉黄芩素组细胞上清肿瘤坏死因子α、白细胞介素1β水平均显著降低(P < 0.05,P < 0.01)。见图6。 2.8 各组FLS细胞状态变化和活性氧含量的检测结果 显微镜下FLS细胞形态显示,空白对照组细胞生长状态良好,轮廓显著为长梭形,呈典型纤维样细胞结构,贴壁牢靠,群体细胞相互连接成网;毒胡萝卜素组大部分细胞改变原有形态,呈多角形,有些轮廓不可见,出现模糊圆团样,群体细胞聚集成堆;随着汉黄芩素给药浓度的增加,长梭形细胞逐渐增多,多角形细胞减少,见图7A。图7B可见,与空白对照组相比,毒胡萝卜素组细胞中平均荧光强度明显增强,表明毒胡萝卜素使细胞内活性氧水平显著提高(P < 0.01)。与毒胡萝卜素组相比,毒胡萝卜素+25 μmol/L汉黄芩素组细胞平均荧光强度几乎无明显变化,提示25 μmol/L汉黄芩素对毒胡萝卜素作用的FLS细胞中活性氧水平无明显影响;毒胡萝卜素+100 μmol/L汉黄芩素组平均荧光强度明显降低(P < 0.01),提示100 μmol/L汉黄芩素降低了毒胡萝卜素作用的FLS细胞中的活性氧水平。荧光显微镜和流式细胞仪检测展现了相同的结果,见图7C,D。 2.9 各组FLS细胞中GRP78、IRE1α、XBP1s、CHOP mRNA表达 与空白对照组相比,毒胡萝卜素组FLS细胞中GRP78、IRE1α、XBP1s、CHOP mRNA表达显著增加(P < 0.05,P < 0.01)。与毒胡萝卜素组相比,毒胡萝卜素+25 μmol/L汉黄芩素组细胞4个mRNA表达水平均无显著变化;毒胡萝卜素+50 μmol/L汉黄芩素组GRP78、XBP1s mRNA表达水平明显降低(P < 0.05),IRE1α、CHOP mRNA表达虽有降低,但差异无显著性意义;毒胡萝卜素+100 μmol/L汉黄芩素组GRP78、IRE1α、XBP1s、CHOP mRNA表达水平均明显降低(P < 0.05,P < 0.01),见图8。以上结果显示汉黄芩素能下调毒胡萝卜素作用FLS细胞中GRP78、IRE1α、XBP1s、CHOP mRNA的相对表达量,且呈现出一定的剂量依赖性。 2.10 各组FLS细胞中GRP78、IRE1α、XBP1s、CHOP蛋白的表达水平 与空白对照组相比,毒胡萝卜素显著上调了FLS细胞中GRP78、IRE1α、XBP1s、CHOP蛋白水平(P < 0.01)。与毒胡萝卜素组相比,毒胡萝卜素+25 μmol/L汉黄芩素组细胞4个蛋白几乎无明显变化;毒胡萝卜素+50 μmol/L汉黄芩素组明显降低了GRP78、IRE1α、CHOP蛋白表达(P < 0.01),XBP1s表达虽也有降低,但差异无显著性意义;毒胡萝卜素+100 μmol/L汉黄芩素组GRP78、IRE1α、XBP1s、CHOP蛋白表达均明显降低(P < 0.05,P < 0.01),见图9。以上结果显示汉黄芩素能下调毒胡萝卜素作用FLS细胞中GRP78、IRE1α、XBP1s、CHOP蛋白的相对表达量,且呈剂量依赖性关系。 "
| [1] NYGAARD G, FIRESTEIN GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16(6):316-333. [2] DAIKH DI. Rheumatoid arthritis: Evolving recognition of a common disease. Best Pract Res Clin Rheumatol. 2022;36(1):101740. [3] SPARKS JA. Rheumatoid Arthritis. Ann Intern Med. 2019;170(1): ITC1-ITC16. [4] BARTOK B, FIRESTEIN GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233-255. [5] ZHANG Q, LIU J, ZHANG M, et al. Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules. 2019;9(12):795. [6] TU J, HONG W, ZHANG P, et al. Ontology and function of fibroblast-like and macrophage-like synoviocytes: how do they talk to each other and can they be targeted for rheumatoid arthritis therapy? Front Immunol. 2018;9:1467. [7] BOTTINI N, FIRESTEIN GS. Duality of fibroblast-like synoviocytes in RA: passive responders andimprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24-33. [8] SCHWARZ DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79-94. [9] LI WY, YANG F, LI X, et al. Stress granules inhibit endoplasmic reticulum stress-mediated apoptosis during hypoxia-induced injury in acute liver failure. World J Gastroenterol. 2023;29(8):1315. [10] RAHMATI M, MOOSAVI MA, MCDERMOTT MF. ER stress: a therapeutic target in rheumatoid arthritis?. Trends Pharmacol Sci. 2018;39(7):610-623. [11] DE SEABRA RODRIGUES DIAS IR, LO HH, ZHANG K, et al. Potential therapeutic compounds from traditional Chinese medicine targeting endoplasmic reticulum stress to alleviate rheumatoid arthritis. Pharmacol Res. 2021;170:105696. [12] DESENY D, BIANCHI E, BAIWIR D, et al. Proteins involved in the endoplasmic reticulum stress are modulated in synovitis of osteoarthritis, chronic pyrophosphate arthropathy and rheumatoid arthritis, and correlate with the histological inflammatory score. Sci Rep. 2020;10(1):14159. [13] LI W, CAO T, LUO C, et al. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl Microbiol Biotechnol. 2020, 104: 6129-6140. [14] KHAN NM, HASEEB A, ANSARI MY, et al. Dataset of effect of Wogonin, a natural flavonoid, on the viability and activation of NF-κB and MAPKs in IL-1β-stimulated human OA chondrocytes. Data Brief. 2017;12:150-155. [15] FAN L, QIU D, HUANG G, et al. Wogonin Suppresses IL-10 Production in B Cells via STAT3 and ERK Signaling Pathway. J Immunol Res. 2020; 2020: 3032425. [16] HUYNH DL, NGAU TH, NGUYEN NH, et al. Potential therapeutic and pharmacological effects of Wogonin: an updated review. Mol Biol Rep. 2020;47(12):9779-9789. [17] BANIK K, KHATOON E, HARSHA C, et al. Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother Res. 2022;36(5):1854-1883. [18] 陆璐, 刘宇婧, 李瑗,等. 基于NLRP3相关炎症反应和肠道黏膜屏障功能探究汉黄芩素治疗溃疡性结肠炎小鼠的作用机制[J]. 中华中医药杂志,2022,37(10):5992-5999. [19] 沈晓庆, 王晶, 李婷君. 汉黄芩素对胶原诱导性关节炎大鼠的治疗作用及对NLRP3炎症小体的影响[J]. 中医药导报,2021,27(8):26-30. [20] 王慧莲, 孟庆良, 李松伟,等. 汉黄芩素通过激活活性氧簇介导的p38MAPK信号通路诱导类风湿关节炎成纤维样滑膜细胞凋亡[J]. 中国骨质疏松杂志,2017,23(7):890-895. [21] 李玉彤, 刘静淑, 李振. ChAT/α7nAChR/核因子κB信号途径在类风湿关节炎发病中的免疫调控[J]. 中国组织工程研究,2023,27(35): 5596-5602. [22] 姬霄霄, 李振, 郝慧琴,等. 防己黄芪汤对CIA大鼠关节PI3K-Akt通路的调控作用研究[J]. 中国免疫学杂志,2023,39(2):291-296. [23] HUANG Y, GUO L, CHITTI R, et al. Wogonin ameliorate complete Freund’s adjuvant induced rheumatoid arthritis via targeting NF‐κB/MAPK signaling pathway. BioFactors. 2020;46(2):283-291. [24] KABALA PA, ANGIOLILLI C, YEREMENKO N, et al. Endoplasmic reticulum stress cooperates with Toll-like receptor ligation in driving activation of rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Res Ther. 2017;19(1):207. [25] COMBE B, RINCHEVAL N, BERENBAUM F, et al. Current favourable 10-year outcome of patients with early rheumatoid arthritis: data from the ESPOIR cohort. Rheumatology(Oxford). 2021;60(11):5073-5079. [26] WANG J, CHEN S, ZHANG J, et al. Scutellaria baicalensis georgi is a promising candidate for the treatment of autoimmune diseases. Front Pharmacol. 2022;13:946030. [27] PARK YJ, YOO SA, KIM WU. Role of endoplasmic reticulum stress in rheumatoid arthritis pathogenesis. J Korean Med Sci. 2014;29(1):2-11. [28] CHOI Y, LEE EG, JEONG JH, et al. 4-Phenylbutyric acid, a potent endoplasmic reticulum stress inhibitor, attenuates the severity of collagen-induced arthritis in mice via inhibition of proliferation and inflammatory responses of synovial fibroblasts. Kaohsiung J Med Sci. 2021;37(7):604-615. [29] LI X, WANG X, WANG Y, et al. Inhibition of transient receptor potential melastatin 7 (TRPM7) channel induces RA FLSs apoptosis through endoplasmic reticulum (ER) stress. Clin Rheumatol. 2014;33: 1565-1574. [30] BETTIGOLE SE, GLIMCHER LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107-138. [31] PENG YW, ZHANG M, HU JK. Non-coding RNAs involved in fibroblast-like synoviocyte functioning in arthritis rheumatoid: From pathogenesis to therapy. Cytokine. 2024;173:156418. [32] LU Q, WANG J, ZHANG X, et al. TXNDC5 protects synovial fibroblasts of rheumatoid arthritis from the detrimental effects of endoplasmic reticulum stress. Intractable Rare Dis Res. 2020;9(1):23-29. [33] MARCINIAK SJ, CHAMBERS JE, RON D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. 2022; 21(2):115-140. [34] MIGLIORANZA SCAVUZZI B, HOLOSHITZ J. Endoplasmic reticulum stress, oxidative stress, and rheumatic diseases. Antioxidants(Basel). 2022; 11(7):1306. [35] KIM EK, KIM Y, YANG JY, et al. Prx1 Regulates Thapsigargin-Mediated UPR Activation and Apoptosis. Genes(Basel). 2022;13(11):2033. [36] HU H, TIAN M, DING C, et al. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol. 2019;9:3083. [37] WISEMAN RL, MESGARZADEH JS, HENDERSHOT LM. Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol Cell. 2022;82(8):1477-1491. [38] SO JS. Roles of endoplasmic reticulum stress in immune responses. Mol Cells. 2018;41(8):705-716. [39] AHMADIANY M, ALAVI-SAMANI M, HASHEMI Z, et al. The Increased RNase Activity of IRE1α in PBMCs from Patients with Rheumatoid Arthritis. Adv Pharm Bull. 2019;9(3):505-509. |
| [1] | Li Jiagen, Chen Yueping, Huang Keqi, Chen Shangtong, Huang Chuanhong. The construction and validation of a prediction model based on multiple machine learning algorithms and the immunomodulatory analysis of rheumatoid arthritis from the perspective of mitophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-15. |
| [2] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [3] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [4] | Qian Kun, Li Ziqing, Sun Shui . Endoplasmic reticulum stress in the occurrence and development of common degenerative bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1285-1295. |
| [5] | Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977. |
| [6] | Zhang Yixuan, Li Dongna, Liu Chunyan. Pathological processes, inflammatory responses, and related biomarkers of periodontitis: a multi-omics analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7601-7610. |
| [7] | Wang Xuepeng, , He Yong, . Effect of insulin-like growth factor family member levels on inflammatory arthritis: a FinnGen biobank-based analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7656-7662. |
| [8] | Wang Tao, Wang Shunpu, Min Youjiang, Wang Min, Li Le, Zhang Chen, Xiao Weiping. Causal relationship between gut microbiota and rheumatoid arthritis: data analysis in European populations based on GWAS data [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7663-7668. |
| [9] | Zhu Jiaping, Gao Bo, Lou Chunbiao, Yang Fengyong, Yang Kun. Monomeric traditional Chinese medicine in the treatment of rheumatoid arthritis: regulation of T cell balance [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6955-6962. |
| [10] | Wang Qifei, Du Xingbin, Kong Jianda. Neural physiological basis and exercise-induced mechanism of central fatigue [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6979-6988. |
| [11] | Li Jiagen, Chen Yueping, Huang Keqi, Chen Shangtong, Huang Chuanhong. Rheumatoid arthritis from the perspective of mitophagy: interaction analysis based on multiple machine learning algorithms [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5595-5607. |
| [12] | Liu Yuan, Qu Yuan, Wan Yakun, Guo Jingyu, Jiang Ping. Transcriptomic analysis and drug prediction of basement membrane-related genes in different traditional Chinese medicine patterns of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5486-5500. |
| [13] | Lin Yixin, Wang Wenyi, Lei Xiaoqing, Ma Dezun, Huang Yanfeng, Fu Changlong, Ye Jinxia. Tougu Xiaotong Capsules for treating arthritis according to the principle of “Same Treatment for Different diseases”: analysis based on integrated pharmacology, molecular docking techniques and molecular dynamics simulation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5093-5101. |
| [14] | Zou Shunyi, Chai yuan, Li Kunjian. Involvement of macrophage polarization in osteoarticular diseases: a visual analysis based on SCI-Expanded information [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5245-5253. |
| [15] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Rheumatoid arthritis and coronary atherosclerosis: data analysis of serum metabolite and inflammatory factor in the European population [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5263-5271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||