Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (5): 958-967.doi: 10.12307/2025.203
Previous Articles Next Articles
Prostaglandin E1 regulates vascular-related factors and protects microcirculatory function during the acute phase of traumatic spinal cord injury
Wang Rongrong1, 2, Huang Yushan1, 2, Li Xiangmiao1, 2, Bai Jinzhu1, 2, 3
- 1School of Rehabilitation Medicine, Capital Medical University, Beijing 100068, China; 2Department of Spine and Spinal Cord Surgery, Beijing Bo’ai Hospital, China Rehabilitation Research Center, Beijing 100068, China; 3Department of Orthopedics, Capital Medical University, Beijing 100069, China
-
Received:2023-11-29Accepted:2024-01-20Online:2025-02-18Published:2024-06-03 -
Contact:Bai Jinzhu, MD, Chief physician, School of Rehabilitation Medicine, Capital Medical University, Beijing 100068, China; Department of Spine and Spinal Cord Surgery, Beijing Bo’ai Hospital, China Rehabilitation Research Center, Beijing 100068, China; Department of Orthopedics, Capital Medical University, Beijing 100069, China -
About author:Wang Rongrong, Master, School of Rehabilitation Medicine, Capital Medical University, Beijing 100068, China; Department of Spine and Spinal Cord Surgery, Beijing Bo’ai Hospital, China Rehabilitation Research Center, Beijing 100068, China
CLC Number:
Cite this article
Wang Rongrong, Huang Yushan, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 regulates vascular-related factors and protects microcirculatory function during the acute phase of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 958-967.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
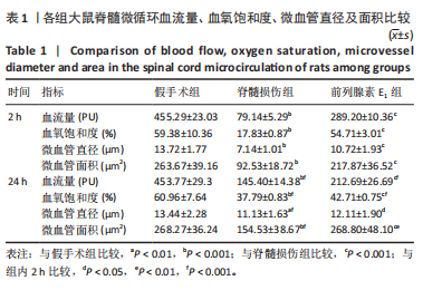
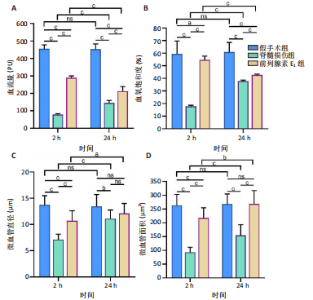
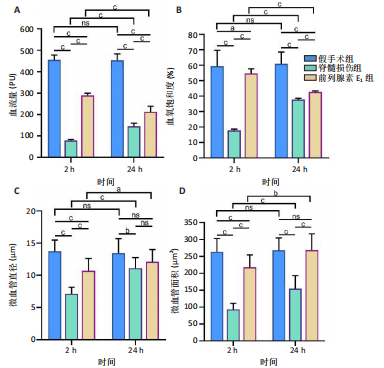
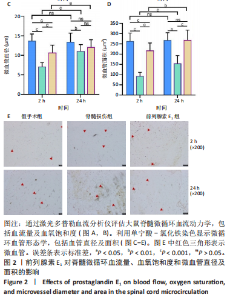
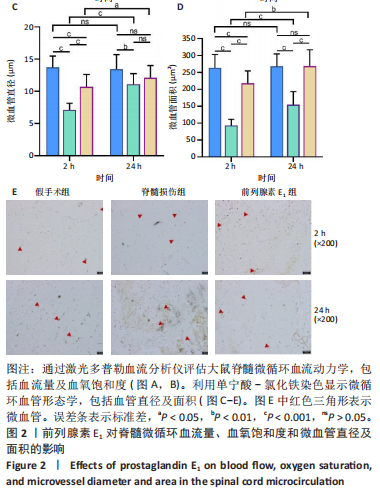
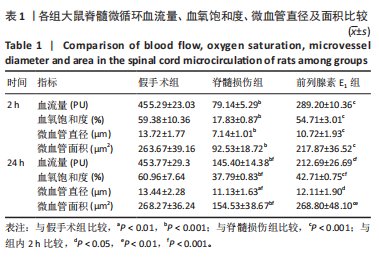
2.1 实验动物数量分析 72只雌性 SD 大鼠随机分为假手术组、脊髓损伤组、前列腺素E1组,每组 24 只。所有大鼠均未死亡,全部进入结果分析。 2.2 前列腺素E1增加微血管直径及面积,改善脊髓微循环血流量和血氧饱和度 为了评估各组大鼠的脊髓微循环血流动力学,此次研究利用激光多普勒血流仪检测脊髓损伤后2,24 h时的脊髓微循环血流量(图2A)及血氧饱和度(图2B)。与对照组大鼠比较,脊髓损伤组大鼠脊髓微循环血流量及血氧饱和度在损伤后显著下降(P < 0.001);在脊髓损伤组内比较发现,与损伤后2 h相比,损伤后24 h脊髓微循环血流量及血氧饱和度更高(P < 0.001)。说明脊髓损伤降低脊髓微循环血流量及血氧饱和度,且脊髓损伤后脊髓微循环血流量及血氧饱和度在24 h有一定程度的自行恢复。与脊髓损伤组大鼠比较,前列腺素E1组大鼠脊髓微循环血流量及血氧饱和度在损伤后2,24 h均有部分恢复(P < 0.001);在前列腺素E1组内比较发现,与损伤后2 h相比,损伤后24 h脊髓微循环血流量及血氧饱和度降低(P < 0.001)。说明脊髓损伤后即刻尾静脉注射前列腺素E1可改善脊髓微循环血流量及血氧饱和度,但药效会随着时间延长减弱。为了评估各组大鼠脊髓微循环的血管形态学,此次研究利用单宁酸-氯化铁染色测定脊髓损伤后2,24 h时的脊髓微循环血管直径及面积(图2C-E)。与假手术组比较,脊髓微循环血管直径在脊髓损伤后2 h(P < 0.001)和24 h(P < 0.01)明显下降,血管面积在脊髓损伤后2,24 h显著降低(P < 0.001);在脊髓损伤组内比较发现,与损伤后2 h比较,损伤后24 h时血管直径和面积有所增加(P < 0.001)。表明脊髓损伤后脊髓微循环的血管直径及面积减小,但是在脊髓损伤后24 h时血管直径和面积有一定恢复。与脊髓损伤组大鼠比较,前列腺素E1组大鼠在损伤后2 h脊髓微循环血管直径(P < 0.001)和面积(P < 0.001)增加,但在损伤后24 h血管直径无统计学差异(P > 0.05),血管面积有所增加(P < 0.001);在前列腺素E1组内比较发现,与损伤后2 h时比较,损伤后24 h时的血管直径(P < 0.05)及面积(P < 0.01)更大。见表1。"
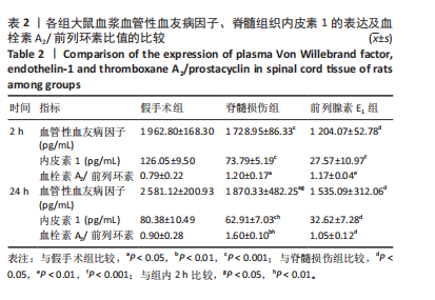
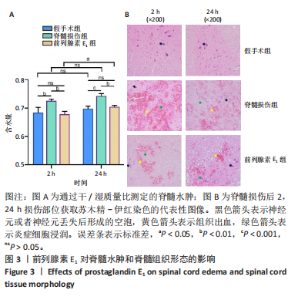
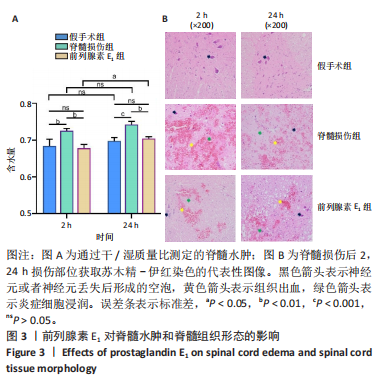
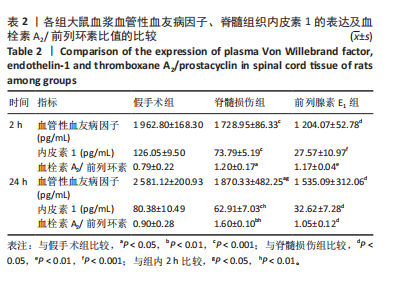
2.3 前列腺素E1减轻脊髓水肿,减少脊髓组织结构的损伤 用干/湿重比法评估脊髓损伤及前列腺素E1对脊髓水肿的影响(图3A)。结果表明,脊髓损伤组含水量在损伤后2,24 h均高于假手术组(2 h:P < 0.01;24 h:P < 0.001);但在脊髓损伤组内比较发现,损伤后2,24 h两个时间点的含水量没有差异(P > 0.05)。静脉注射前列腺素E1可减轻脊髓损伤后脊髓水肿,降低脊髓含水量(2 h:P < 0.01;24 h:P < 0.01);在前列腺素E1组内比较发现,与损伤后2 h时比较,损伤后24 h时脊髓含水量更高(P < 0.05)。组织病理学检查显示,对照组脊髓结构完整,没有出血、坏死或者炎症浸润;而脊髓损伤组大鼠损伤2 h后,脊髓结构破坏,神经元损伤,灰质发生出血,血管的周围组织有炎症细胞浸润现象;24 h后,脊髓灰质出血严重,部分神经元坏死,血管周围组织的炎症细胞浸润现象加重。脊髓损伤后使用前列腺素E1干预,相对于脊髓损伤组,出血、神经元损伤和炎症有所减轻。见图3B。 2.4 前列腺素E1下调血浆血管性血友病因子、脊髓组织中内皮素1的表达及血栓素A2/前列环素比值 为了评估脊髓损伤后血管调节因子变化及前列腺素E1对血管调节因子的影响,此次研究利用ELISA法定量测定脊髓损伤后2,24 h时的血管调节因子含量变化(表2)。ELISA检测结果显示,与对照组大鼠比较,脊髓损伤组大鼠血浆血管性血友病因子(2 h:P < 0.001;24 h:P < 0.05)、脊髓组织中内皮素1(2 h: P < 0.001;24 h:P < 0.001)及血栓素A2/ 前列环素比值(2 h:P < 0.05;24 h:P < 0.01)在损伤后2,24 h时显著增加。在脊髓损伤组内比较发现,与损伤后2 h比较,损伤后24 h血浆血管性血友病因子含量(P < 0.05)及脊髓组织中血栓素A2/ 前列环素比值(P < 0.01)更高,而脊髓组织中内皮素1含量下降(P < 0.01)。与脊髓损伤组大鼠比较,前列腺素E1组大鼠在损伤后2,24 h血浆血管性血友病因子(2 h:P < 0.05;24 h:P < 0.05)、脊髓组织中内皮素1表达(2 h:P < 0.001;24 h:P < 0.05)及血栓素A2/ 前列环素比值(2 h:P < 0.01;24 h:P < 0.05)降低。在前列腺素E1组内比较发现,2个时间点的血浆血管性血友病因子含量(P > 0.05)、脊髓组织内皮素1含量(P > 0.05)及血栓素A2/ 前列环素比值(P > 0.05)均无统计学差异。"

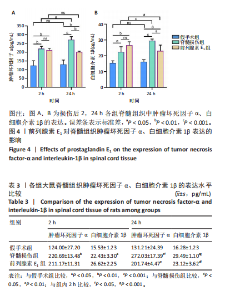
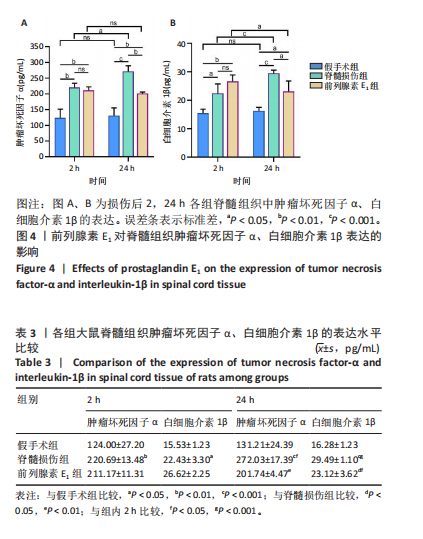
ELISA检测结果显示,与假手术组比较,脊髓损伤组脊髓组织中肿瘤坏死因子α(2 h:P < 0.01;24 h:P < 0.001)、白细胞介素1β(2 h:P < 0.05;24 h:P < 0.001)的质量浓度在2,24 h时均显著增加。在脊髓损伤组内比较发现,与损伤后2 h相比,损伤后24 h时肿瘤坏死因子α(P < 0.05)、白细胞介素1β(P < 0.001)质量浓度更高。与脊髓损伤组大鼠比较,前列腺素E1组脊髓组织中肿瘤坏死因子α(P < 0.01)、白细胞介素1β(P < 0.05)质量浓度在损伤后24 h明显减少,损伤后2 h无统计学差异(P > 0.05)。前列腺素E1组内比较发现,脊髓组织肿瘤坏死因子α质量浓度在2个时间点无统计学差异(P > 0.05),但是脊髓组织白细胞介素1β质量浓度在损伤后24 h时下降(P < 0.05)。"

| [1] KARSY M, HAWRYLUK G. Modern Medical Management of Spinal Cord Injury. Curr Neurol Neurosci Rep. 2019;19(9):65-67. [2] SOFRONIEW MV. Dissecting Spinal Cord Regeneration. Nature (London). 2018;557(7705):343-350. [3] JANI RH, PRABHU AV, ZHOU JJ, et al. Citation Analysis of the Most Influential Articles On Traumatic Spinal Cord Injury. J Spinal Cord Med. 2020;43(1):31-38. [4] SANDEAN D. Management of Acute Spinal Cord Injury: A Summary of the Evidence Pertaining to the Acute Management, Operative and Non-Operative Management. World J Orthop. 2020;11(12): 573-583. [5] SEKHON LHS, FEHLINGS MG. Epidemiology, Demographics, and Pathophysiology of Acute Spinal Cord Injury. Spine (Philadelphia, Pa. 1976). 2001;26(24):S2-S12. [6] XIN W, QIANG S, JIANING D, et al. Human Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate Blood-Spinal Cord Barrier Disruption Via the Timp2/Mmp Pathway After Acute Spinal Cord Injury. Mol Neurobiol. 2021;58(12):6490-6504. [7] TATOR CH, FEHLINGS MG. Review of the Secondary Injury Theory of Acute Spinal Cord Trauma with Emphasis On Vascular Mechanisms. J Neurosurg. 1991;75(1):15-26. [8] ALIZADEH A, DYCK SM, KARIMI-ABDOLREZAEE S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10:282. [9] WANG X, JIANG C, ZHANG Y, et al. The Promoting Effects of Activated Olfactory Ensheathing Cells On Angiogenesis After Spinal Cord Injury through the Pi3K/Akt Pathway. Cell Biosci. 2022;12(1):23. [10] MARCOLINA A, VU K, ANNASWAMY TM. Lumbar Spinal Stenosis and Potential Management with Prostaglandin E1 Analogs. Am J Phys Med Rehabil. 2021;100(3):297-302. [11] XIE X, LU W, CHEN Y, et al. Prostaglandin E1 Alleviates Cognitive Dysfunction in Chronic Cerebral Hypoperfusion Rats by Improving Hemodynamics. Front Neurosci. 2019;13:549. [12] LIU L, ZHANG H, SHI Y, et al. Prostaglandin E1 Improves Cerebral Microcirculation through Activation of Endothelial Nos and Grpch1. J Mol Neurosci. 2020;70(12):2041-2048. [13] HAMAMOTO Y, OGATA T, MORINO T, et al. Prostaglandin E1 Analog Increases Spinal Cord Blood Flow at the Point of Compression During and After Experimental Spinal Cord Injury. Spinal Cord. 2010;48(2): 149-153. [14] COHEN J. Statistical Power Analysis for the Behavioral Sciences (Revised Edition). Laurence Erlbaum Associates: Hillsdale, NJ, USA. 1987. [15] SUZUKI T, OBATA R, INOUE T, et al. Intravenous Lipo-Prostaglandin E1 Administration for Patients with Acute Central Retinal Artery Occlusion. BMJ Open Ophthalmol. 2022;7(1):e001014. [16] SHEN J, CAO M, ZHOU T, et al. PgE1 Triggers Nrf2/Ho-1 Signal Pathway to Resist Hemin-Induced Toxicity in Mouse Cortical Neurons. Ann Transl Med. 2021;9(8):634. [17] WANG J, TANG S, XIE Y, et al. Lipid Microsphere-Coated PgE1 Improves Peritoneal Transport and Reduces Inflammation in Peritoneal Dialysis: A Randomized Clinical Pilot Trial. Semin Dial. 2021;34(3):235-244. [18] LI J, WANG B, WANG Y, et al. Therapeutic Effect of Liposomal Prostaglandin E(1) in Acute Lower Limb Ischemia as an Adjuvant to Hybrid Procedures. Exp Ther Med. 2013;5(6):1760-1764. [19] MACHADO-ALBA JE, MACHADO-DUQUE ME. Use of Intravenous Alprostadil in Patients with Severe Critical Ischemia of the Lower Limbs. Vascular. 2019;27(3):318-323. [20] ERAIFEJ J, NNADI C, GANAU M. Early and Ultra-Early Surgical Decompression for Acute Spinal Cord Injury: Bracing for the Winds of Change. Eur Spine J. 2022;31(7):1691-1692. [21] YE J, WEN Z, WU T, et al. Single-Cell Sequencing Reveals the Optimal Time Window for Anti-Inflammatory Treatment in Spinal Cord Injury. Adv Biol (Weinh). 2023;7(10):e2300098. [22] KAWATA K, MORIMOTO T, OHASHI T, et al. Experimental Study of Acute Spinal Cord Injury: A Histopathological Study. No Shinkei Geka. 1993;21(1):45-51. [23] WALLACE MC, TATOR CH, FRAZEE P. Relationship Between Posttraumatic Ischemia and Hemorrhage in the Injured Rat Spinal Cord as Shown by Colloidal Carbon Angiography. Neurosurgery. 1986;18(4):433-439. [24] ROOZBEHI A, JOGHATAIE MT, MEHDIZADEH M, et al. The Effects of Cyclosporin-a On Functional Outcome and Axonal Regrowth Following Spinal Cord Injury in Adult Rats. Acta Med Iran. 2012;50(4):226-232. [25] YAO C, CAO X, YU B. Revascularization After Traumatic Spinal Cord Injury. Front Physiol. 2021;12:631500. [26] MARCUS ML, HEISTAD DD, EHRHARDT JC, et al. Regulation of Total and Regional Spinal Cord Blood Flow. Circ Res. 1977;41(1):128-134. [27] QIN L, WANG Y, XIE Z, et al. The Role of Et-1 in Early Cerebral Microcirculation Changes After Subarachnoid Hemorrhage. J Healthc Eng. 2022;2022:4592986. [28] FREYERMUTH-TRUJILLO X, SEGURA-URIBE JJ, SALGADO-CEBALLOS H, et al. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells. 2022;11(17):2692. [29] JIANG T, QIN T, GAO P, et al. Sirt1 Attenuates Blood-Spinal Cord Barrier Disruption After Spinal Cord Injury by Deacetylating P66Shc. Redox Biol. 2023;60:102615. [30] YUAN X, WU Q, WANG P, et al. Exosomes Derived From Pericytes Improve Microcirculation and Protect Blood-Spinal Cord Barrier After Spinal Cord Injury in Mice. Front Neurosci. 2019;13:319. [31] MANZ XD, BOGAARD HJ, AMAN J. Regulation of Vwf (Von Willebrand Factor) in Inflammatory Thrombosis. Arterioscler Thromb Vasc Biol. 2022;42(11):1307-1320. [32] ARISZ RA, de VRIES JJ, SCHOLS SEM, et al. Interaction of Von Willebrand Factor with Blood Cells in Flow Models: A Systematic Review. Blood Adv. 2022;6(13):3979-3990. [33] ISHIHARA J, ISHIHARA A, STARKE RD, et al. The Heparin Binding Domain of Von Willebrand Factor Binds to Growth Factors and Promotes Angiogenesis in Wound Healing. Blood. 2019;133(24):2559-2569. [34] LI X, LUO D, HOU Y, et al. Sodium Tanshinone Iia Silate Exerts Microcirculation Protective Effects Against Spinal Cord Injury in Vitro and in Vivo. Oxid Med Cell Longev. 2020;2020:3949575. [35] IMIG JD. Eicosanoid Blood Vessel Regulation in Physiological and Pathological States. Clin Sci (Lond). 2020;134(20):2707-2727. [36] DEMOPOULOS HB, FLAMM ES, PIETRONIGRO DD, et al. The Free Radical Pathology and the Microcirculation in the Major Central Nervous System Disorders. Acta physiol Scand Suppl. 1980;492:91-119. [37] BRAUNE S, KÜPPER J, JUNG F. Effect of Prostanoids On Human Platelet Function: An Overview. Int J Mol Sci. 2020;21(23):9020. [38] MAMO YA, ANGUS JA, ZIOGAS J, et al. The Role of Voltage-Operated and Non-Voltage-Operated Calcium Channels in Endothelin-Induced Vasoconstriction of Rat Cerebral Arteries. Eur J Pharmacol. 2014;742:65-73. [39] A JC, LI ZY, LONG QF, et al. Mir-379-5P Improved Locomotor Function Recovery After Spinal Cord Injury in Rats by Reducing Endothelin 1 and Inhibiting Astrocytes Expression. Eur Rev Med Pharmacol Sci. 2019;23(22):9738-9745. [40] JIN LY, LI J, WANG KF, et al. Blood-Spinal Cord Barrier in Spinal Cord Injury: A Review. J Neurotrauma. 2021;38(9):1203-1224. [41] ZHOU ZL, XIE H, TIAN XB, et al. Microglial depletion impairs glial scar formation and aggravates inflammation partly by inhibiting STAT3 phosphorylation in astrocytes after spinal cord injury. Neural Regen Res. 2023;18(6):1325-1331. [42] LI Z, ZHAO J, LI S, et al. Blocking the Egfr/P38/Nf-Κb Signaling Pathway Alleviates Disruption of Bscb and Subsequent Inflammation After Spinal Cord Injury. Neurochem Int. 2021;150:105190. [43] CAI X, ZHAO J, LU Y, et al. The Microenvironment Following Oxygen Glucose Deprivation/Re-Oxygenation-Induced Bscb Damage in Vitro. Brain Res Bull. 2018;143:171-180. [44] XU Z, XU W, CHEN X, et al. Study On Vascular Remodeling, Inflammatory Response, and their Correlations in Acute Spinal Cord Injury in Rats. Zhongguo xiufu chongjian waike zazhi. 2020;34(11):1429-1437. [45] YUN J. Interleukin-1Β Induces Pericyte Apoptosis Via the Nf-Κb Pathway in Diabetic Retinopathy. Biochem Biophys Res Commun. 2021;546: 46-53. [46] SHARMA HS. A Combination of Tumor Necrosis Factor-Alpha and Neuronal Nitric Oxide Synthase Antibodies Applied Topically Over the Traumatized Spinal Cord Enhances Neuroprotection and Functional Recovery in the Rat. Ann N Y Acad Sci. 2010;1199:175-185. [47] SHARMA HS, WINKLER T, STÅLBERG E, et al. Topical Application of Tnf-Alpha Antiserum Attenuates Spinal Cord Trauma Induced Edema Formation, Microvascular Permeability Disturbances and Cell Injury in the Rat. Acta Neurochir Suppl. 2003;86:407-413. [48] AUBERT JD, JUILLERAT-JEANNERET L. Endothelin-Receptor Antagonists Beyond Pulmonary Arterial Hypertension: Cancer and Fibrosis. J Med Chem. 2016;59(18):8168-8188. [49] LIU L, ZHANG H, SHI Y, et al. Prostaglandin E1 Improves Cerebral Microcirculation through Activation of Endothelial Nos and Grpch1. J Mol Neurosci. 2020;70(12):2041-2048. [50] LIU B, ZHANG S, XIONG X, et al. Lipo‑Prostaglandin E1 Modifies Cognitive Impairment in Rats with Vascular Cognitive Impairment by Promoting Angiogenesis Via the Vegf/Vegfr Pathway. Mol Med Rep. 2017;16(3):3117-3124. [51] NAIMUSHIN YA, MAZUROV AV. Von Willebrand Factor Can Support Platelet Aggregation Via Interaction with Activated Gpiib-Iiia and Gpib. Platelets. 2004;15(7):419-425. [52] ZDROJOWY K, ADAMIEC R, CZARNACKI M. New Aspects of Prostaglandin E1 Therapeutic Influence in Thrombo-Angiitis Obliterans. Pol Arch Med Wewn. 2002;108(5):1071-1077. [53] ITOH Y, YASUI T, KAKIZAWA H, et al. The Therapeutic Effect of Lipo PgE1 On Diabetic Neuropathy-Changes in Endothelin and Various Angiopathic Factors. Prostaglandins Other Lipid Mediat. 2001;66(3):221-234. [54] LIU HJ, MA JW, QIAO ZY, et al. Study of Molecular Mechanism of Prostaglandin E1 in Inhibiting Coronary Heart Disease. Mol Biol Rep. 2013;40(12):6701-6708. [55] ZHANG L, WANG X, SI H. Synergistic Anti-Inflammatory Effects and Mechanisms of the Combination of Resveratrol and Curcumin in Human Vascular Endothelial Cells and Rodent Aorta. J Nutr Biochem. 2022;108:109083. [56] VIRTUOSO A, DE LUCA C, KORAI SA, et al. Neuroinflammation and glial activation in the central nervous system: a metabolic perspective. Neural Regen Res. 2023;18(5):1025-1026. [57] LIU S, GAN N, XIE J, et al. Clinical Evaluation of Alprostadil Combined with Nimodipine in Treatment of Cerebral Vasospasm After Subarachnoid Hemorrhage in Elderly Patients. Pak J Med Sci. 2023; 39(3):682-686. [58] TSAI CH, HUANG PJ, LEE IT, et al. Correction for: Endothelin-1-Mediated Mir-Let-7G-5P Triggers Interlukin-6 and Tnf-Alpha to Cause Myopathy and Chronic Adipose Inflammation in Elderly Patients with Diabetes Mellitus. Aging (Albany NY). 2023;15(1):287. [59] GÜR FM, BILGIÇ S. A Synthetic Prostaglandin E1 Analogue, Misoprostol, Ameliorates Paclitaxel-Induced Oxidative Damage in Rat Brain. Prostaglandins Other Lipid Mediat. 2022;162:106663. [60] 李雪梅. 前列地尔注射液单次及双次给药对大鼠心肌缺血再灌注损伤的保护作用对比研究[D].长春:吉林大学,2016. [61] 曹先德, 郑慧敏, 万春平. 前列腺素E1联合缺血预处理对肾部分切除术肾缺血再灌注损伤的机制研究[J]. 交通医学,2019;33(2): 119-122, 125. [62] 戴伟娟, 朱凡河, 张国安, 等. 前列腺素E1对大鼠全脑缺血-再灌注损伤细胞凋亡及Caspase-3表达的影响[J]. 济宁医学院学报, 2017;40(2):98-102. |
| [1] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [3] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [4] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [5] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [6] | Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838. |
| [7] | Wang Tong, Zheng Yu, Jia Chengming, Yang Hu, Zhang Guangfei, Ji Yaoyao. Action mechanism of Gegenmaqi prescription in treatment of periarthritis of shoulder combined with type 2 diabetes based on TCMSP database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7669-7678. |
| [8] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [9] | Xu Biao, Dong Yuzhen, Lu Tan. Effect of dihydroquercetin on the expression of inflammatory response markers in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6843-6850. |
| [10] | Hu Shujuan, Liu Dang, Ding Yiting, Liu Xuan, Xia Ruohan, Wang Xianwang. Ameliorative effect of walnut oil and peanut oil on atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6482-6488. |
| [11] | Geng Longyu, Sheng Li, Bai Shuo, Gao Beiyao, Ge Ruidong, Jiang Shan . Role and molecular mechanism of pyroptosis in motor system diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5695-5703. |
| [12] | Guan Zhenjie, Li Wenyuan, Geng Rui, Wang Ying. Regulatory mechanisms of the corticospinal tract after spinal cord injury: combined therapeutic strategies targeting transcription factors and signaling pathways [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5158-5170. |
| [13] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Rheumatoid arthritis and coronary atherosclerosis: data analysis of serum metabolite and inflammatory factor in the European population [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5263-5271. |
| [14] | Hu Shujuan, Cheng Ping, Zhang Xiao, Ding Yiting, Liu Xuan, Pu Rui, Wang Xianwang. Effects of different exercise interventions on carboxylesterase 1 and inflammatory factors in skeletal muscle of type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 269-278. |
| [15] | Yang Yike, Ren Yafeng, Li Bing, Shang Wenya, Huang Jing, Guo Jia, Liu Huiyao . Regulatory mechanisms and therapeutic strategies for cellular autophagy after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3885-3896. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||