Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (1): 136-146.doi: 10.12307/2024.722
Previous Articles Next Articles
Mammalian pluripotent stem cells: effects on creating disease models, pathogenesis, drug discovery and personalized treatment
Xu Wenqiang1, 2, Chen Haolin3, Yan Chang1, Xu Tao1, Xie Yabin1, Li Xueling2
- 1Inner Mongolia Key Laboratory of Hypoxic Translational Medicine, School of Medical Technology and Anesthesia, School of Basic Medicine and Forensic Medicine, Baotou Medical College, Baotou 014060, Inner Mongolia Autonomous Region, China; 2State Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock, Inner Mongolia University, Hohhot 010010, Inner Mongolia Autonomous Region, China; 3First Affiliated Hospital of Dalian Medical University, Dalian 116000, Liaoning Province, China
-
Received:2023-08-22Accepted:2023-10-14Online:2025-01-08Published:2024-05-18 -
Contact:Xie Yabin, Associate professor, Master’s supervisor, Inner Mongolia Key Laboratory of Hypoxic Translational Medicine, School of Medical Technology and Anesthesia, School of Basic Medicine and Forensic Medicine, Baotou Medical College, Baotou 014060, Inner Mongolia Autonomous Region, China Co-corresponding author: Li Xueling, Professor, Doctoral supervisor, Master’s supervisor, State Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock, Inner Mongolia University, Hohhot 010010, Inner Mongolia Autonomous Region, China -
About author:Xu Wenqiang, PhD, Lecturer, Master’s supervisor, Inner Mongolia Key Laboratory of Hypoxic Translational Medicine, School of Medical Technology and Anesthesia, School of Basic Medicine and Forensic Medicine, Baotou Medical College, Baotou 014060, Inner Mongolia Autonomous Region, China; State Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock, Inner Mongolia University, Hohhot 010010, Inner Mongolia Autonomous Region, China -
Supported by:Scientific Research Foundation Project of Baotou Medical College, No. BYJJ-ZRQM 202215, (to XWQ); Baotou Medical College “Bud Plan Project”, No. HLJH202312 (to XWQ), HLJH202320 (to XYB); Scientific Research Foundation Project of Baotou Medical College, No. BBJJ201804 (to XYB); Inner Mongolia Natural Science Foundation Project, No. 2021LHMS08022 (to XYB); Inner Mongolia University Scientific Research Project, No. NJZZ19189 (to XYB)
CLC Number:
Cite this article
Xu Wenqiang, Chen Haolin, Yan Chang, Xu Tao, Xie Yabin, Li Xueling. Mammalian pluripotent stem cells: effects on creating disease models, pathogenesis, drug discovery and personalized treatment[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 136-146.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
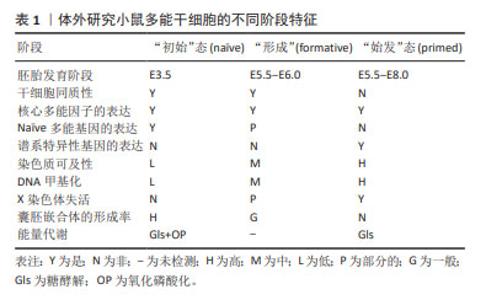
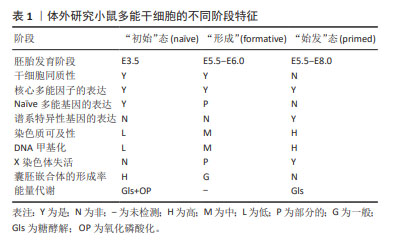
2.1 多能干细胞多能性的定义 根据经典理论,从小鼠囊胚的内细胞团中获得的胚胎干细胞被定义为“初始”(na?ve)态,特征体现在独特的外部信号依赖性、转录活性、表观遗传和代谢表型[5]。当干细胞来自植入后的上胚层时,它们被称为上胚层干细胞,并被定义为“始发”(primed)态多能干细胞,代表na?ve后续的多能发育阶段,Primed态多能干细胞在功能上不同于na?ve[6-7]。最近,KINOSHITA团队[8]提出并验证了一种处于na?ve态和primed态之间的形成态(formative)的存在。他们从小鼠和人类胚胎中分离培养出了形成态多能干细胞,并通过嵌合体实验和多组学分析验证了形成态多能干细胞的生物学特征。 大量的研究证据表明,多能干细胞的多能性不是一种独特的细胞状态,而是连续阶段的发展进程,所有这些细胞都能够进行自我更新和分化,与体内发育的妊娠阶段相对应,且具有不同的代谢、线粒体和表观遗传特征[9-10]。表1总结了体外培养的小鼠多能干细胞不同阶段的特征。 "
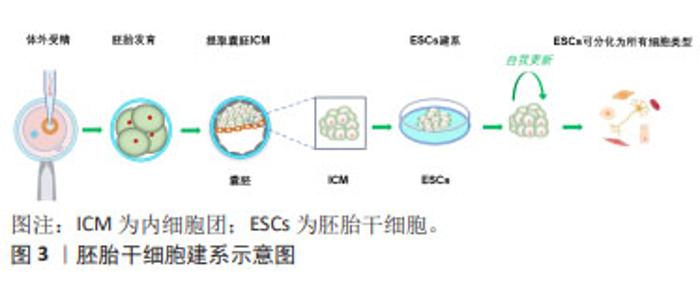
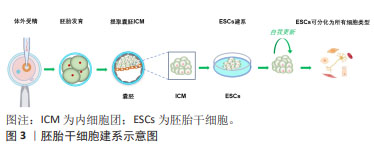
2.2 多能干细胞的研究进展 数十年来,干细胞技术不断推陈出新,为生命科学研究打开了崭新的局面。随着不断扩展和深入研究,多能干细胞分类也在发生变化。到目前为止,在文章对干细胞研究进行系统回顾后,总结出了6种基本的多能干细胞。 2.2.1 胚胎干细胞 胚胎干细胞来源于胚胎着床前胚泡期(囊胚)的内细胞团,它们具有分化成身体所有细胞类型的能力,见图3。1981年,胚胎干细胞第一次成功地从小鼠囊胚的内细胞团获得,并可在体外培养并长期保持多能性[2-3]。小鼠胚胎干细胞被定义为“na?ve”多能性,具有无偏分化和嵌合体贡献潜力[5]。体外培养所需的特定条件,如饲养层的共培养以及后来的化学成分确定的无饲养层培养体系,使胚胎干细胞表现出无限的增殖能力和未分化的潜能状态。"
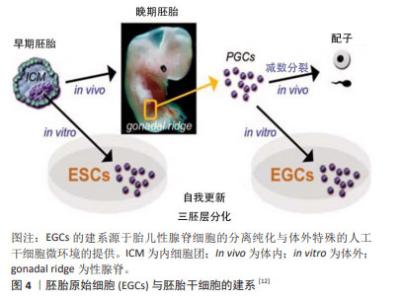
1998年是生命科学研究历史上具有划时代意义的一年,THOMSON等[1]从人类囊胚的内细胞团获得并建立人胚胎干细胞系,这些人胚胎干细胞具备正常核型,并表达高水平端粒酶活性,和灵长类胚胎干细胞特征的细胞表面标记物,但不表征早期谱系分化标记物,长期体外培养的人胚胎干细胞仍具备形成滋养层和三胚层衍生物的潜能[5]。然而,与小鼠内细胞团来源的胚胎干细胞不同的是,人类或灵长类动物胚胎干细胞特征相当于小鼠上胚层干细胞,属于“primed”多能态,具有偏分化能力和有限的嵌合体贡献潜力[5]。人胚胎干细胞的应用价值在于为基础研究和再生医学领域提供重要的细胞工具,具体包括:用于探究胚胎正常的发育机制,并揭示出生缺陷和疾病的原因;研究胚胎发育早期细胞凋亡、分化、诱变、免疫排斥和细胞衰老的理想模型材料;用于治疗药物的疗效和毒性检测;诱导形成人类脏器、软骨组织、神经元和血管等组织;定向分化为特定细胞类型,为疾病和残疾的治疗提供可再生的替代细胞和组织。但同时,人胚胎干细胞细胞也有诱发畸胎瘤和免疫排斥的风险,这是必须面对和解决的问题。 2.2.2 胚胎生殖细胞 除囊胚内细胞团之外,胚胎的原始生殖细胞(primordial germ cells,PGCs)同样可以用于多能干细胞的建立[11-12],见图4。早在1992年,MATSUI等[11]从E10.5-E12.5的小鼠胚胎中分离原始生殖细胞,在添加了膜相关铁因子(steel factor,SF)、白血病抑制因子(leukemia inhibitory factor,LIF)和碱性成纤维生长因子(basic fibroblast growth factor,bFGF)的培养体系中,实现了原始生殖细胞在体外的连续增殖;胚胎干细胞的各项检测标准表明原始生殖细胞符合胚胎干细胞的基本特征。原始生殖细胞的长期培养及其重编程为多能的胚胎干细胞对生殖细胞生物学和畸胎瘤的诱导具有重要意义。"
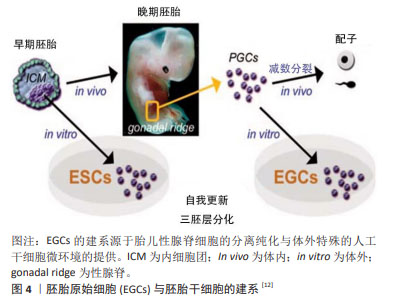
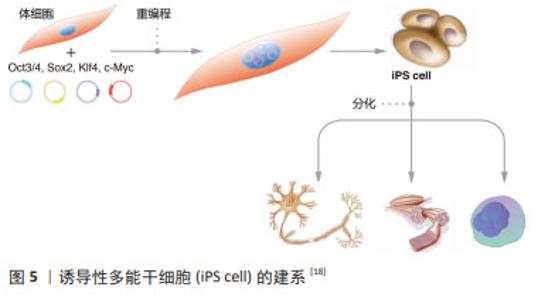
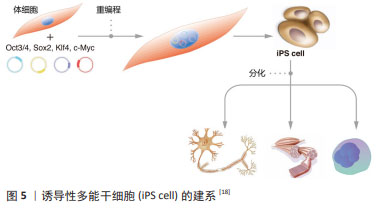
来源于原始生殖细胞的人胚胎干细胞被称为人胚胎生殖细胞(human embryonic germ cells,hEGCs)。SHAMBLOTT等[13]于1998年首次建立了人胚胎生殖细胞细胞系,即从受精后5-9周的人胎儿分离得到含有原始生殖细胞的性腺脊和肠系膜,利用添加了重组的人碱性成纤维细胞生长因子(recombinant human basic fibroblast growth factor,rhbFGF)、重组人白血病抑制因子(recombinant human leukemia inhibitory factor,rhLIF)及毛喉素的培养基,在小鼠STO成纤维细胞饲养层上进行培养,从而建立了最早的人胚胎生殖干细胞系。随后,有3个科研团队相继报道获得可以在体外自我更新、保持未分化状态,并可分化为三胚层衍生物的人胚胎生殖细胞[14-16]。这些人类原始生殖细胞来源的培养物符合多潜能干细胞的标准,与胚胎生殖细胞非常相似,它们与人胚胎干细胞细胞一起丰富了人类多能干细胞的定义。人胚胎生殖细胞在细胞分化和细胞移植研究中具有潜在的应用前景,已成为生命科学和医学研究的前沿热点[13,17]。 2.2.3 诱导多能干细胞(induced pluripotent stem cells,iPSCs) 来源于哺乳动物囊胚内细胞团或胚胎原始生殖细胞的胚胎干细胞,在保持多能性的同时具有无限生长的能力[2-3,13]。人胚胎干细胞的这些特性给疾病机制的研究、有效且安全药物的筛选以及各种疾病和损伤患者的治疗带来了希望。然而,人类胚胎的使用却面临着阻碍人胚胎干细胞应用的伦理争议,而且,可有效应用的患者或疾病特异性的胚胎干细胞的获得难度极大。因此,规避这些问题的一种方法是通过重编程诱导体细胞发生去分化,从而产生干细胞多能性。在2012年,诺贝尔生理学或医学奖授予TAKAHASHI的诱导性多能干细胞,其团队建立了在Oct3/4,Sox2,Klf4和c-Myc四因子作用下将成年小鼠和人的成纤维细胞重新编程为类胚胎干细胞的方案[4],机制如图5所示[18]。他们最初利用反转录病毒转导技术将24个候选基因用于重新编程小鼠成纤维细胞,随后这些候选基因逐渐被精确到Oct4,Sox2,c-Myc和Klf4等4个转录因子[4]。紧接着,这项技术被成功地应用于人成纤维细胞诱导产生诱导性多能干细胞[19]。与此同时,YU等[20]研究发现Oct4,Sox2,Nanog和Lin28足以重新编程人类细胞,其中Oct4和Sox2似乎是必不可少的,另外两个基因强烈(Nanog)或适度(Lin28)的影响重新编程的效率。"
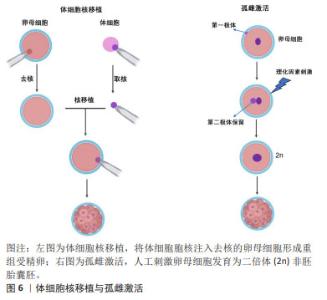
由于可以从患者自身采集体细胞,所以诱导性多能干细胞克服了胚胎干细胞的免疫排斥和伦理限制的缺点。更重要的是,诱导性多能干细胞表现出许多胚胎干细胞类似的特征,包括细胞表型、多能marker表达、染色体端粒特征以及形成拟胚体、嵌合体或畸胎瘤等能力[21]。然而,诱导性多能干细胞的缺点也逐渐引起了人们的担忧,这在一定程度上限制了诱导性多能干细胞的应用开发。首先,基因传递所用到的病毒载体可能导致多种病毒整合到受体细胞的基因组中,引起细胞的遗传异常乃至肿瘤发生;其次,由人成纤维细胞重编程为诱导性多能干细胞的效率极低( < 0.02%);再次,癌基因Myc作为重编程因子,激活沉默的Myc基因容易诱导诱导性多能干细胞成为癌细胞[22];最后,诱导性多能干细胞的重编程过程和随后的长期体外培养容易导致这些细胞的遗传不稳定和表观遗传异常。这些弊端引起了人们对诱导性多能干细胞未来应用的担忧,因此有必要进行更深入的研究,以了解重新编程过程,并需要深入研究这些基因组和表观基因组变化的生物学结构。 2.2.4 胚胎干细胞样细胞 胚胎干细胞样细胞是指能够表现出与胚胎干细胞相似的特性,但不是来自胚胎的细胞,胚胎干细胞样细胞制备方法主要包括体细胞核移植(somatic cell nuclear transfer,SCNT),孤雌生殖,以及新生或成体生殖细胞来源的干细胞系,见图6。它们通常与胚胎干细胞具有相同的关键特征,如自我更新和分化成多种细胞类型的能力。 SCNT是指将体细胞的细胞核移植到去核的卵母细胞中形成重构的受精卵,后者被激活并培养至囊胚期可用于干细胞建系,或者通过胚胎移植技术移植到母体子宫进行动物克隆。除受精之外,SCNT或称为克隆技术,也可以赋予体细胞全能性。GURDON博士[23]最早于1962年首次证明可以通过SCNT从分化的青蛙体细胞中克隆动物。30多年后,第一只克隆哺乳动物多利羊诞生了[24]。在SCNT过程中,受体卵母细胞的胞质中遗留的因子可对供体细胞核进行重编程,从而使其重新获得多能性和多向分化潜能。此外,基因编辑技术也被运用与SCNT-胚胎干细胞建系,LEE等[25]使用CRISPR/cas9介导的基因靶向产生了B2M纯合子敲除体细胞核移植诱导胚胎干细胞(SCNT-ESC)系。B2MKO细胞株在细胞表面不表达hla-1分子,而具有多能性和向三胚层分化的能力。"
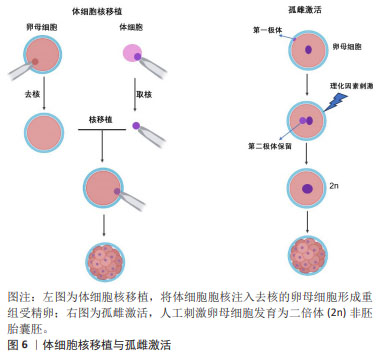
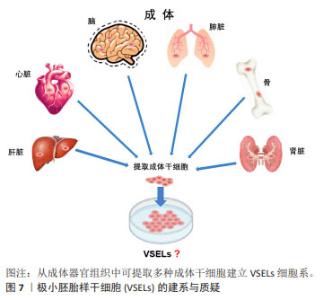
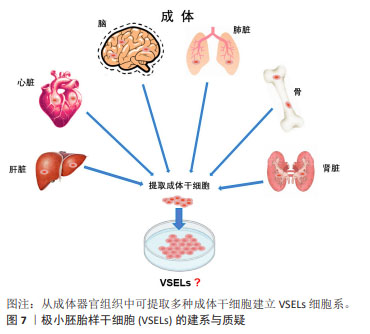
孤雌生殖是指在没有受精的情况下激活卵子的胚胎发育。哺乳动物卵母细胞可通过人工刺激发育为二倍体非胚胎囊胚,并可从囊胚内细胞团中获得孤雌生殖干细胞。JU等[26]利用从同一小鼠卵母细胞供体分离的体细胞核进行核移植和孤雌激活,建立了符合胚胎干细胞标准的多能干细胞系。在灵长类动物中,由于遗传缺陷影响了正常的胎盘形成,孤雌生殖无法长成可存活的胎儿[27]。然而,孤雌生殖干细胞仍具备一定的多能性,可发育为视网膜色素上皮样细胞、肌样和骨样细胞、神经元细胞和肝细胞。 从新生或成体睾丸组织或卵巢组织中分离并进行类胚胎干细胞的培养,可建立符合干细胞标准的胚胎干细胞样细胞系。研究发现新生和成年小鼠睾丸中的精原干细胞或雄性生殖系干细胞表型上与胚胎干细胞相似,同时具备体外三胚层分化和畸胎瘤形成能力,并且显示出生殖系贡献与传递[28]。GUAN等[28]从成年小鼠睾丸中分离精原干细胞,在体外的特殊培养条件下获得胚胎干细胞的特性。这些细胞能够在体外自发分化为三胚层的衍生物,并在免疫缺陷小鼠中产生畸胎瘤。当注入早期囊胚时,精原干细胞有助于各种器官的发育,并表现出种系传播[29]。 存在于卵巢中的雌性生殖系干细胞,早先被认为可能保留了产生多能干细胞的能力,如WANG等[30]从卵巢中提取的雌性生殖干细胞(female germline stem cells,FGSCs)在干细胞多能基因表达和分化潜力方面表现出胚胎干细胞类似的特性。GONG等[31]通过培养10周龄B6D2F1 (C57BL/6×DBA2)雌性小鼠卵巢细胞,建立了符合干细胞标准的胚胎干细胞样细胞细胞系。然而,近年来关于卵巢干细胞的多能特性出现了很大争议,甚至有新的证据表明FGSCs并不存在。尽管如此,这些新技术为研究生殖细胞生物学提供了可能性,并为个性化的再生应用夯实了基础。最近,利用非生殖系干细胞生产人工生殖细胞的研究为不孕不育症的治疗开辟了新的途径。 GOLESTANEH等[32]首次报道了通过成人睾丸细胞建立的人胚胎干细胞样细胞,他们从人类器官供体获得睾丸组织,并将其从干细胞龛中移至添加有适当的生长因子和试剂的胚胎干细胞培养基,发现持续的培养可使睾丸生殖细胞重编程为多能干细胞。人胚胎干细胞样细胞的建立为成人自体干细胞治愈疾病和干细胞生物技术与医学的应用提供了可能性。 此外,从成年生物体的骨髓中分离出的间充质干细胞或多能基质细胞是人体内分布最广泛的细胞之一[33],由于具备骨细胞、软骨细胞、脂肪细胞和其他细胞系的分化潜能,被认为具有多能干细胞特征[34]。间充质干细胞不仅具有产生异位骨组织的能力,而且可以转分化为上皮细胞和神经外胚层的谱系,更重要的是,间充质干细胞具备通过分泌可溶性因子改变组织微环境的能力,为间充质干细胞的广泛治疗功效提供了基础。 2.2.5 极小胚胎样干细胞 在再生医学领域,成体干细胞尽管克服了胚胎干细胞的伦理问题和其他技术问题,在某些方面可能成为胚胎干细胞的潜在替代者,然而有研究表明,成体干细胞具有多能干细胞部分属性,可能的机制包括干细胞的转分化或去分化,或者干细胞与不同谱系细胞的融合[35]。大量研究表明,在成人组织中存在多潜能干细胞,包括极小胚胎样干细胞、多能成体干细胞、间充质干细胞和骨髓分离的成人多系诱导细胞等[36-37]。 在这些细胞中,最具特征的是在成体器官,如成人性腺、脐带血/组织和骨髓中被分离和鉴定的极小胚胎样干细胞[38]。极小胚胎样干细胞为成体器官组织来源的干细胞,在器官发生的早期沉积,并可作为组织定向干细胞的来源,见图7。尽管目前没有被广泛接受,甚至后来有人对极小胚胎样干细胞的客观存在及其多能性提出质疑,但是已有研究揭示了极小胚胎样干细胞在再生医学和细胞治疗中的巨大潜力:极小胚胎样干细胞与胚胎干细胞相同的多能性特征,包括Oct4和Nanog启动子区域开放的染色质结构和体外的三胚层分化能力[39]。不同的是,极小胚胎样干细胞的嵌合体和畸胎瘤形成能力不足,致瘤风险较低,而且一般情况下极小胚胎样干细胞处于静息状态,代谢活性较低,可作为组织定向干细胞的备份,在受伤发生时进入细胞周期,为组织再生和动态平衡做出贡献[40]。极小胚胎样干细胞的缺点是体外培养不像胚胎干细胞那样容易,这可能与一些发育关键基因的甲基化修饰有关。 值得注意的是,关于极小胚胎样干细胞的真实性和分化能力曾遭受怀疑。有研究报道称在小鼠骨髓中并不能找到任何已经报道的具备干细胞潜能,特别是造血潜能的极小胚胎样干细胞,他们发现“极小胚胎样干细胞”并不表达Oct4,而且不能分化为血细胞[41]。然而,面对质疑,KUCIA等[37]指出该研究团队没有遵循先前发表在Current Cytometry Protocols上的极小胚胎样干细胞分离详细方案,从而导致极小胚胎样干细胞没有得到充分的分离和纯化。"
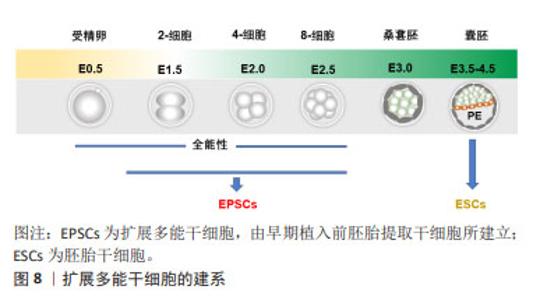
2.2.6 扩展多能干细胞 胚胎干细胞和诱导性多能干细胞由于具备分化为机体所有类型细胞的潜能被认为具有多能性,而且在过去的数十年,它们在胚胎早期发育机制的研究和干细胞治疗的探索与应用方面发挥着积极的作用。然而,胚胎干细胞和诱导性多能干细胞并不具备原始受精卵的“全能性”特征,即同时形成胚胎组织和胚外组织[42]。人胚胎干细胞的研究一直致力于模拟体内胚胎的发生过程,然而,之前所用primed态人胚胎干细胞相当于小鼠早期植入后的上胚层干细胞[43],并且缺乏类似滋养外胚(trophectoderm,TE)和原始内胚层(primitive endoderm,PE)的细胞。因此,他们不能完全概括人类早期胚胎发育的谱系相互作用。 从早期植入前胚胎(4细胞-8细胞)提取干细胞,通过抑制植入前胚胎中运行的谱系分化相关途径,可建立扩展多能干细胞系,见图8。扩展多能干细胞具有强大的自我更新能力,并在体外培养或嵌合体实验中产生胚胎和胚胎外细胞系[44-45]。扩展多能干细胞由于具备全能多能性,又被称为全能干细胞。小鼠来源的扩展多能干细胞的建立是从早期植入前胚胎提取干细胞,通过抑制在植入前囊胚胚胎中运行的分子信号途径,如促进卵裂球分化的关键分子通路而建立的[46]。"
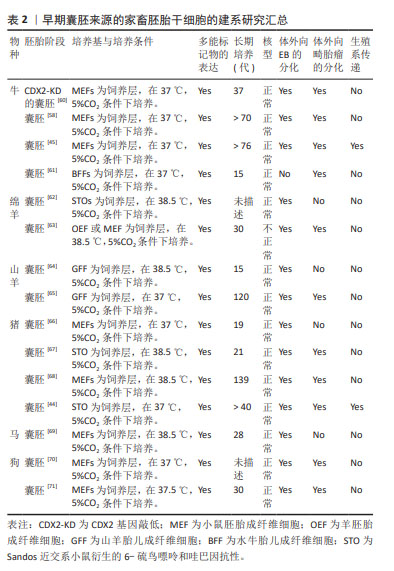
值得注意的是,一些重要的化学小分子也被用于扩展多能干细胞的体外制备。SHEN等[47]利用剪接体抑制剂Pladienolide B成功驱动体外培养的小鼠胚胎干细胞从多能态向全能态的转变,并实现了全能干细胞产生胚胎和胚外细胞系的双向发育潜力的长期保持。HU等[48]利用维甲酸类似物TTNPB、1-氮杂苯丙酮和激酶阻滞剂WS6共3个小分子的组合成功实现了体外培养的小鼠全能干细胞的化学诱导和长期维持。XU等[49]发现一种化学“鸡尾酒”能够从小鼠2-细胞胚胎和扩展多能干细胞中衍生出全能样干细胞,这些细胞表现出与2-细胞小鼠胚胎相同的特征,并在单细胞水平上具备胚胎和胚胎外发育潜力。YANG等[50]则通过化学诱导着丝粒周围异染色质的重塑以及全能特异性的宽H3K4me3结构域的重建,从表观遗传角度促进了小鼠胚胎干细胞从多能到全能的转变。 扩展多能干细胞的建系方法相对降低了从囊胚期胚胎中建立干细胞的异质性。更重要的是,扩展多能干细胞允许高效的基因组编辑,这使其具备很好的发展潜力,为其在广泛的生物技术应用和农业研究领域提供了重要基础。 2.3 家畜多能干细胞的研究进展与应用 回顾多能干细胞的研究史和发展过程,容易发现各种类型多能干细胞的建立基本上都会经历从小鼠或大鼠等模型动物开始,继而推广到人和其他哺乳动物。从家畜中已获得的多能干细胞,包括胚胎干细胞、诱导性多能干细胞、生殖细胞谱系的多能干细胞和扩展多能干细胞,为动物繁殖育种、基因工程、疾病模型、新药筛选和野生濒危动物保护开辟了新的途径,具有发展畜牧业和创造人类福利的巨大潜力。 2.3.1 胚胎干细胞 小鼠胚胎干细胞成功获得之后,研究者尝试在猪、牛、羊、兔子等家畜动物中建立囊胚内细胞团来源的干细胞系[51-53]。然而,在家畜物种建立胚胎干细胞的进程是极其缓慢的。从20世纪80年代开始,经过数十年的努力,绵羊、猪和牛等家畜物种来源的早期胚胎类胚胎干细胞才被陆续报道;然而,具体的多能干细胞多能特征表明,这些细胞多数处于primed状态,且在体外只能维持数十代,猪、牛、绵羊、水牛的类胚胎干细胞[54-55],不能保持强大的自我更新能力,而且通常在畸胎瘤和嵌合体试验中无法完成3个胚层的分化。这可能源于家畜物种和啮齿类在细胞谱系形成的个体发育方面的差异,以及控制多能性的分子途径的差异。直到2008年,3i方法首次被成功用于大鼠真正胚胎干细胞的分离和培养,从而为家畜多能干细胞的建立提供了有价值的参考[56]。3i法是一种添加与胚胎干细胞诱导分化信号通路相关的3种抑制剂的培养方案,即通过在培养基微环境中添加CHIR99021(GSK3激酶抑制剂)、PD184352(ERK1/2激酶抑制剂)和SU5402(成纤维细胞生长因子酪氨酸激酶受体抑制剂)3种小分子,促进符合干细胞特征的家畜多能干细胞的成功建系[57]。 牛胚胎干细胞的研究始于1996年,为了获得牛胚胎干细胞,已经进行了大量的研究。然而,所获得的这些干细胞难以满足多能干细胞的全部标准,体外长期培养中多能性难以保持;三胚层衍生效率低下;体外和体内实验中发育潜力有限。直到2018年,BOGLIOTTI等[58]首次报道了基本符合干细胞标准的牛胚胎干细胞的成功建立。他们在MEF饲养层上,利用特定的mTeSR1基础培养基(不含生长因子)、经典的WNT信号通路抑制剂IWR1和bFGF组成的CTFR培养体系,在体外成功地实现了符合干细胞特征的牛胚胎干细胞长期培养;而且这些干细胞可作为核移植供体产生正常的囊胚,为基因组选择和基因组编辑提供了可能性。HAN等[59]研究发现组蛋白甲基化修饰酶抑制剂MLL1与PD0325901和CHIR99021(2i)相结合,可促进IVF来源的牛囊胚的发育。文章总结了早期囊胚来源的家畜胚胎干细胞最新研究成果[44-45,58,60-71],并展示在表2中。 "
| [1] THOMSON JA, ITSKOVITZ-ELDOR J, SHAPIRO SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391): 1145-1147. [2] MARTIN GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634-7638. [3] EVANS MJ, KAUFMAN MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154-156. [4] TAKAHASHI K, YAMANAKA S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. [5] NICHOLS J, SMITH A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4(6):487-492. [6] BRONS IG, SMITHERS LE, TROTTER MW, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448(7150): 191-195. [7] TESAR PJ, CHENOWETH JG, BROOK FA, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448(7150):196-199. [8] KINOSHITA M, BARBER M, MANSFIELD W, et al. Capture of mouse and human stem cells with features of formative pluripotency. Cell Stem Cell. 2021;28(3):453-471.e458. [9] SPERBER H, MATHIEU J, WANG Y, et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol. 2015;17(12):1523-1535. [10] HARVEY AJ, RATHJEN J GARDNER DK. Metaboloepigenetic regulation of pluripotent stem cells. Stem Cells Int. 2016;2016:1816525. [11] MATSUI Y, ZSEBO K, HOGAN BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70(5):841-847. [12] TURNPENNY L, SPALLUTO CM, PERRETT RM, et al. Evaluating human embryonic germ cells: concord and conflict as pluripotent stem cells. Stem Cells. 2006;24(2):212-220. [13] SHAMBLOTT MJ, AXELMAN J, WANG S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95(23):13726-13731. [14] LIU S, LIU H, PAN Y, et al. Human embryonic germ cells isolation from early stages of post-implantation embryos. Cell Tissue Res. 2004;318(3):525-531. [15] PARK JH, KIM SJ, LEE JB, et al. Establishment of a human embryonic germ cell line and comparison with mouse and human embryonic stem cells. Mol Cells. 2004;17(2):309-315. [16] TURNPENNY L, BRICKWOOD S, SPALLUTO CM, et al. Derivation of human embryonic germ cells: an alternative source of pluripotent stem cells. Stem Cells. 2003;21(5):598-609. [17] SHAMBLOTT MJ, AXELMAN J, LITTLEFIELD JW, et al. Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc Natl Acad Sci U S A. 2001;98(1): 113-118. [18] KARAGIANNIS P, NAKAUCHI A, YAMANAKA S. Bringing induced pluripotent stem cell technology to the bedside. JMA J. 2018;1(1):6-14. [19] PARK IH, ZHAO R, WEST JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141-146. [20] YU J, VODYANIK MA, SMUGA-OTTO K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858): 1917-1920. [21] MARION RM, STRATI K, LI H, et al. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4(2): 141-154. [22] TAKAHASHI K, ICHISAKA T, YAMANAKA S. Identification of genes involved in tumor-like properties of embryonic stem cells. Methods Mol Biol. 2006; 329:449-458. [23] GURDON JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10: 622-640. [24] WILMUT I, SCHNIEKE AE, MCWHIR J, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810-813. [25] LEE OH, LEE S, PARK M, et al. Generation of a B2M homozygous knockout human somatic cell nuclear transfer-derived embryonic stem cell line using the CRISPR/Cas9 system. Stem Cell Res. 2021;59:102643. [26] JU JY, PARK CY, GUPTA MK, et al. Establishment of stem cell lines from nuclear transferred and parthenogenetically activated mouse oocytes for therapeutic cloning. Fertil Steril. 2008;89(5 Suppl):1314-1323. [27] FUNDELE RH, NORRIS ML, BARTON SC, et al. Temporal and spatial selection against parthenogenetic cells during development of fetal chimeras. Development. 1990;108(1):203-211. [28] GUAN K, NAYERNIA K, MAIER LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440(7088):1199-1203. [29] KANATSU-SHINOHARA M, INOUE K, LEE J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119(7):1001-1012. [30] WANG H, JIANG M, BI H, et al. Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol. 2014;6(2):164-171. [31] GONG SP, LEE ST, LEE EJ, et al. Embryonic stem cell-like cells established by culture of adult ovarian cells in mice. Fertil Steril. 2010;93(8):2594-2601, 2601. e2591-e2599. [32] GOLESTANEH N, KOKKINAKI M, PANT D, et al. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18(8):1115-1126. [33] FUKUCHI Y, NAKAJIMA H, SUGIYAMA D, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004; 22(5):649-658. [34] LI Z, HU X, ZHONG JF. Mesenchymal stem cells: characteristics, function, and application. Stem Cells Int. 2019;2019:8106818. [35] WAGERS AJ, WEISSMAN IL. Plasticity of adult stem cells. Cell. 2004;116(5): 639-648. [36] BELTRAMI AP, CESSELLI D, BERGAMIN N, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood. 2007;110(9):3438-3446. [37] KUCIA M, RECA R, CAMPBELL FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857-869. [38] PARTE S, BHARTIYA D, TELANG J, et al. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011;20(8): 1451-1464. [39] ZUBA-SURMA EK, WU W, RATAJCZAK J, et al. Very small embryonic-like stem cells in adult tissues-potential implications for aging. Mech Ageing Dev. 2009;130(1-2):58-66. [40] RATAJCZAK MZ, ZUBA-SURMA EK, SHIN DM, et al. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008;43(11):1009-1017. [41] MIYANISHI M, MORI Y, SEITA J, et al. Do pluripotent stem cells exist in adult mice as very small embryonic stem cells? Stem Cell Reports. 2013;1(2):198-208. [42] CHOI YJ, LIN CP, RISSO D, et al. Deficiency of microRNA miR-34a expands cell fate potential in pluripotent stem cells. Science. 2017;355(6325):eaag1927. [43] NAKAMURA T, OKAMOTO I, SASAKI K, et al. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature. 2016; 537(7618):57-62. [44] GAO X, NOWAK-IMIALEK M, CHEN X, et al. Establishment of porcine and human expanded potential stem cells. Nat Cell Biol. 2019;21(6):687-699. [45] ZHAO L, GAO X, ZHENG Y, et al. Establishment of bovine expanded potential stem cells. Proc Natl Acad Sci U S A. 2021. doi: 10.1073/pnas.2018505118. [46] YANG J, RYAN DJ, LAN G, et al. In vitro establishment of expanded-potential stem cells from mouse pre-implantation embryos or embryonic stem cells. Nat Protoc. 2019;14(2):350-378. [47] SHEN H, YANG M, LI S, et al. Mouse totipotent stem cells captured and maintained through spliceosomal repression. Cell. 2021;184(11):2843-2859.e2820. [48] HU Y, YANG Y, TAN P, et al. Induction of mouse totipotent stem cells by a defined chemical cocktail. Nature. 2023;617(7962):792-797. [49] XU Y, ZHAO J, REN Y, et al. Derivation of totipotent-like stem cells with blastocyst-like structure forming potential. Cell Res. 2022;32(6):513-529. [50] YANG M, YU H, YU X, et al. Chemical-induced chromatin remodeling reprograms mouse ESCs to totipotent-like stem cells. Cell Stem Cell. 2022; 29(3):400-418.e413. [51] BOROVIAK T, STIRPARO GG, DIETMANN S, et al. Single cell transcriptome analysis of human, marmoset and mouse embryos reveals common and divergent features of preimplantation development. Development. 2018;145(21):dev167833. [52] VASSILIEV I, NOTTLE MB. Isolation and culture of porcine embryonic stem cells. Methods Mol Biol. 2013;1074:85-95. [53] HONDA A, HIROSE M, OGURA A. Basic FGF and Activin/Nodal but not LIF signaling sustain undifferentiated status of rabbit embryonic stem cells. Exp Cell Res. 2009;315(12):2033-2042. [54] CIBELLI JB, STICE SL, GOLUEKE PJ, et al. Transgenic bovine chimeric offspring produced from somatic cell-derived stem-like cells. Nat Biotechnol. 1998; 16(7):642-646. [55] ZHU SX, SUN Z, ZHANG JP. Ovine (Ovis aries) blastula from an in vitro production system and isolation of primary embryonic stem cells. Zygote. 2007;15(1):35-41. [56] LI P, TONG C, MEHRIAN-SHAI R, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135(7):1299-1310. [57] MURRAY JT, CAMPBELL DG, MORRICE N, et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J. 2004;384(Pt 3):477-488. [58] BOGLIOTTI YS, WU J, VILARINO M, et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci U S A. 2018;115(9):2090-2095. [59] HAN X, XIANG J, LI C, et al. MLL1 combined with GSK3 and MAP2K inhibition improves the development of in vitro-fertilized embryos. Theriogenology. 2020; 146:58-70. [60] WU X, SONG M, YANG X, et al. Establishment of bovine embryonic stem cells after knockdown of CDX2. Sci Rep. 2016;6:28343. [61] VERMA OP, KUMAR R, NATH A, et al. In vivo differentiation potential of buffalo (Bubalus bubalis) embryonic stem cell. In Vitro Cell Dev Biol Anim. 2012;48(6):349-358. [62] PILICHI S, ROCCA S, DATTENA M, et al. Sheep embryonic stem-like cells engrafted into sheep femoral condyle osteochondral defects: 4-year follow-up. BMC Vet Res. 2018;14(1):213. [63] ZHAO Y, LIN J, WANG L, et al. Derivation and characterization of ovine embryonic stem-like cell lines in semi-defined medium without feeder cells. J Exp Zool A Ecol Genet Physiol. 2011;315(10):639-648. [64] KUMAR DE A, MALAKAR D, AKSHEY YS, et al. Isolation and characterization of embryonic stem cell-like cells from in vitro produced goat (Capra hircus) embryos. Anim Biotechnol. 2011;22(4):181-196. [65] BEHBOODI E, BONDAREVA A, BEGIN I, et al. Establishment of goat embryonic stem cells from in vivo produced blastocyst-stage embryos. Mol Reprod Dev. 2011;78(3):202-211. [66] CHA HJ, YUN JI, HAN NR, et al. Generation of embryonic stem-like cells from in vivo-derived porcine blastocysts at a low concentration of basic fibroblast growth factor. Reprod Domest Anim. 2018;53(1):176-185. [67] HOU DR, JIN Y, NIE XW, et al. Derivation of porcine embryonic stem-like cells from in vitro-produced blastocyst-stage embryos. Sci Rep. 2016;6:25838. [68] ZHANG M, WANG C, JIANG H, et al. Derivation of novel naive-like porcine embryonic stem cells by a reprogramming factor-assisted strategy. FASEB J. 2019; 33(8):9350-9361. [69] LI X, ZHOU SG, IMREH MP, et al. Horse embryonic stem cell lines from the proliferation of inner cell mass cells. Stem Cells Dev. 2006;15(4):523-531. [70] TOBIAS IC, BROOKS CR, TEICHROEB JH, et al. Small-molecule induction of canine embryonic stem cells toward naïve pluripotency. Stem Cells Dev. 2016;25(16): 1208-1222. [71] VAAGS AK, ROSIC-KABLAR S, GARTLEY CJ, et al. Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells. 2009;27(2):329-340. [72] WANG L, DUAN E, SUNG LY, et al. Generation and characterization of pluripotent stem cells from cloned bovine embryos. Biol Reprod. 2005; 73(1):149-155. [73] GEORGE A, SHARMA R, SINGH KP, et al. Production of cloned and transgenic embryos using buffalo (Bubalus bubalis) embryonic stem cell-like cells isolated from in vitro fertilized and cloned blastocysts. Cell Reprogram. 2011;13(3):263-272. [74] MUZAFFAR M, SELOKAR NL, SINGH KP, et al. Equivalency of buffalo (Bubalus bubalis) embryonic stem cells derived from fertilized, parthenogenetic, and hand-made cloned embryos. Cell Reprogram. 2012;14(3):267-279. [75] SINGH KP, KAUSHIK R, GARG V, et al. Expression pattern of pluripotent markers in different embryonic developmental stages of buffalo (Bubalus bubalis) embryos and putative embryonic stem cells generated by parthenogenetic activation. Cell Reprogram. 2012;14(6):530-538. [76] MUNOZ M, RODRIGUEZ A, DE FRUTOS C, et al. Conventional pluripotency markers are unspecific for bovine embryonic-derived cell-lines. Theriogenology. 2008;69(9):1159-1164. [77] KUMAR D, ANAND T, VIJAYALAKSHMY K, et al. Transposon mediated reprogramming of buffalo fetal fibroblasts to induced pluripotent stem cells in feeder free culture conditions. Res Vet Sci. 2019;123:252-260. [78] PILLAI VV, KEI TG, REDDY SE, et al. Induced pluripotent stem cell generation from bovine somatic cells indicates unmet needs for pluripotency sustenance. Anim Sci J. 2019;90(9):1149-1160. [79] XIANG J, WANG H, ZHANG Y, et al. LCDM medium supports the derivation of bovine extended pluripotent stem cells with embryonic and extraembryonic potency in bovine-mouse chimeras from iPSCs and bovine fetal fibroblasts. FEBS J. 2021;288(14):4394-4411. [80] SEBO J PARENT B. Human, nonhuman, and chimeric research: considering old issues with new research. Hastings Cent Rep. 2022;52 Suppl 2:S29-S33. [81] YAMANAKA S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27(4):523-531. [82] CIERVO Y, NING K, JUN X, et al. Advances, challenges and future directions for stem cell therapy in amyotrophic lateral sclerosis. Mol Neurodegener. 2017;12(1):85. [83] DIMOS JT, RODOLFA KT, NIAKAN KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218-1221. [84] GURNEY ME, PU H, CHIU AY, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772-1775. [85] ARNOLD ES, LING SC, HUELGA SC, et al. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci U S A. 2013;110(8):E736-E745. [86] LUND RD, WANG S, KLIMANSKAYA I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8(3):189-199. [87] KELAVA I, LANCASTER M A. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev Biol. 2016;420(2):199-209. [88] DE MASI C, SPITALIERI P, MURDOCCA M, et al. Application of CRISPR/Cas9 to human-induced pluripotent stem cells: from gene editing to drug discovery. Hum Genomics. 2020;14(1):25. [89] LI LB, BONINI NM. Roles of trinucleotide-repeat RNA in neurological disease and degeneration. Trends Neurosci. 2010;33(6):292-298. [90] ADER M, TANAKA EM. Modeling human development in 3D culture. Curr Opin Cell Biol. 2014;31:23-28. [91] SALMASI S, KALASKAR DM, YOON WW, et al. Role of nanotopography in the development of tissue engineered 3D organs and tissues using mesenchymal stem cells. World J Stem Cells. 2015;7(2):266-280. [92] STABLER CT, LECHT S, MONDRINOS MJ, et al. Revascularization of decellularized lung scaffolds: principles and progress. Am J Physiol Lung Cell Mol Physiol. 2015; 309(11):L1273-L1285. [93] BAPTISTA LS, KRONEMBERGER GS, CÔRTES I, et al. Adult stem cells spheroids to optimize cell colonization in scaffolds for cartilage and bone tissue engineering. Int J Mol Sci. 2018;19(5):1285. [94] PEPER J, KOWNATZKI-DANGER D, WENINGER G, et al. Caveolin3 stabilizes McT1-mediated lactate/proton transport in cardiomyocytes. Circ Res. 2021;128(6):e102-e120. [95] CHEN H, CROSS AC, THAKKAR A, et al. Selective linkage of mitochondrial enzymes to intracellular calcium stores differs between human-induced pluripotent stem cells, neural stem cells, and neurons. J Neurochem. 2021; 156(6):867-879. [96] SHARMA A, MCKEITHAN WL, SERRANO R, et al. Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nature protocols. 2018;13(12):3018-3041. [97] SALMAN MM, KITCHEN P, YOOL AJ, et al. Recent breakthroughs and future directions in drugging aquaporins. Trends Pharmacol Sci. 2022;43(1):30-42. [98] MACHIRAJU P GREENWAY SC. Current methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes. World J Stem Cells. 2019;11(1):33-43. [99] KHATEB S, JHA S, BHARTI K, et al. Cell-based therapies for age-related macular degeneration. Adv Exp Med Biol. 2021;1256:265-293. |
| [1] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [2] | Deng Li, Liu Yang, Wang Hui, Yang Qiu, Dong Mingqing. Transcription factor NKX2.1 promotes differentiation of induced pluripotent stem cells into lung stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7790-7796. |
| [3] | Liu Lu, Zhong Chang, Yu Xin, Ren Chenyuan, Gong Yangyang, Zhou Ping, Wang Yingbin. Academic progress and clinical application of in vitro synthetic microenvironment to promote maturation of human pluripotent stem cell-derived cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7856-7862. |
| [4] | Xiong Tinglin, Zhang Lisha, Wang Dewei, Cao Haiping, Yang Yan. Effect of culture time in vitro on maturity of induced pluripotent stem cell-derived cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5304-5310. |
| [5] | Zhao Wen, Bi Yulin, Fu Xuyang, Duan Hongmei, Yang Zhaoyang, Li Xiaoguang. Stem cell therapy for amyotrophic lateral sclerosis: cell source, number, modification, and administration route [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4083-4090. |
| [6] | Zheng Ankai, Liu Ruiming, Xiang Qiuling. Application of stem cells in endothelialization of small-diameter blood vessel prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 120-127. |
| [7] | Ma Yangguang, Zhang Yayong, Meng Mingyao, Jin Zhihao, Li Yingming, Huang Yaoxuan, Han Shen, Li Yaxiong. Application and mechanism of induced pluripotent stem cells in inherited heart disease models [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4072-4078. |
| [8] | Cui Shengnan, Liu Chuanguo, Yang Wenqing, Zheng Zhijuan, Zhang Dan. Effects and mechanisms of calycosin on endothelial differentiation of human induced pluripotent stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 3031-3036. |
| [9] | Hu Wei, Xing Jian, Chen Guangxin, Chen Zee, Zhao Yi, Qiao Dan, Ouyang Kunfu, Huang Wenhua. Myocardial patch: cell sources, improvement strategies, and optimal production methods [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2723-2730. |
| [10] | Wen Tinghao, Li Yuandi, He Keke, Song Wenqian, Wang Xianbin, Gao Jie, Su Min, Hu Rong. Wnt signaling pathway is involved in differentiation of embryonic stem cells into thymic epithelial progenitor cells together with autoimmune regulators [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 1996-2001. |
| [11] | Zheng Xiaohan, Feng Xiaoli, Hu Lan, Gao Shijun, Wei Yanzhao, Huang Ting, Sun Shengtong, Wei Xufang, Wang Tan, Zhao Zhenqiang. Macrophage migration inhibitory factor promotes the differentiation of human embryonic stem cells into ventral midbrain dopaminergic neural progenitor cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5348-5356. |
| [12] | Huang Guicai, Luo Yehao, Jiang Huirong, Li Yuan, Mao Dewen, Guan Zhijie. Clinical therapeutic prospect and current research status of stem cells for liver diseases [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(19): 3091-3098. |
| [13] | Zheng Xiaohan, Wei Yanzhao, Huang Ting, Wei Xufang, Sun Shengtong, Wang Tan, Zhao Zhenqiang. Role of macrophage migration inhibitory factor for stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(15): 2395-2403. |
| [14] | Huang Wenjun, Wang Jie, Zhou Yafei, Li Huan, Jiang Congshan, Zhang Yanmin, Zhou Rui. Establishment and identification of an efficient protocol for differentiation of endothelial cells from human induced pluripotent stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1553-1559. |
| [15] | Wei Yanzhao, Zheng Xiaohan, Gao Shijun, Huang Ting, Wei Xufang, Chen Xinxu, Zhao Zhenqiang. Expression of autocrine macrophage migration inhibitory factor and its receptors of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 34-41. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||