Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (32): 5225-5230.doi: 10.12307/2024.492
Previous Articles Next Articles
Near-infrared fluorescence imaging for intraoperative neuroimaging: current applications and future development
Gao Wenping1, Zhang Zhihua2, Han Fei1, Hao Weiguo1
- 1Department of Orthopedics, Shanxi Veterans Hospital, Taiyuan 030031, Shanxi Province, China; 2Shanxi Health Vocational College, Jinzhong 030619, Shanxi Province, China
-
Received:2023-08-28Accepted:2023-09-20Online:2024-11-18Published:2023-12-29 -
Contact:Gao Wenping, Department of Orthopedics, Shanxi Veterans Hospital, Taiyuan 030031, Shanxi Province, China -
About author:Gao Wenping, Associate chief physician, Department of Orthopedics, Shanxi Veterans Hospital, Taiyuan 030031, Shanxi Province, China
CLC Number:
Cite this article
Gao Wenping, Zhang Zhihua, Han Fei, Hao Weiguo. Near-infrared fluorescence imaging for intraoperative neuroimaging: current applications and future development[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5225-5230.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
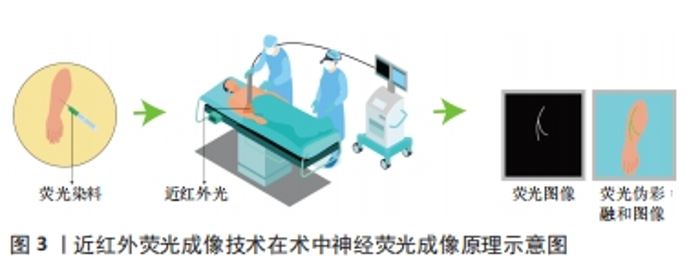
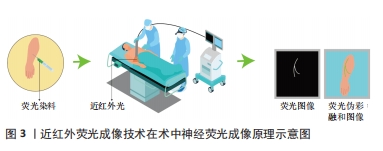
2.1 近红外荧光成像技术 近年来,荧光引导外科手术作为一种非侵入性技术不断发展,已经成为外科手术和精准医学研究的热门领域。这种技术的基本原理是通过静脉或局部注射将可区分目标组织和其他组织的显影剂注入患者体内,然后利用外部设备激发显影剂产生荧光信号。外部传感器用于接收和处理不同组织的荧光信号,并形成实时图像传输到在屏幕上。这种图像能够准确显示细胞内某一基因或生物大分子特性的表达情况,并记录展示这些动态过程。研究者们通过信背比(signal to background ratio,SBR)进行记录和分析,技术成功的标准是SBR值大于1,且SBR值越大,显示效果越明显。 近红外光是波段位于650-2 500 nm内的电磁波,目前在生物分析研究和生物医学诊断方面得到广泛的应用[16]。人的肉眼不能直接看到近红外光,需要使用特殊的光学成像系统,也就是近红外荧光成像系统才能观察到。一般研究者们会把近红外荧光染料当做显影剂来使用。当手术视野中照射到某种波长的近红外光时,手术室视野中就会发出另一种波长的近红外光。荧光染料的位置可以通过捕捉这种发射出来的光来精确判定。利用手术视野中的近红外光,当研究者们在活体细胞、组织或器官上标记出近红外荧光染料时,就可以对目标组织或结构进行精确定位。 目前的光学成像技术主要在可见光区域(400-650 nm)和近红外一区(Near-infraredⅠ, NIR-Ⅰ,650-900 nm)光谱区域进行[17]。然而,当在可见光区域成像时,它们会受严重的组织散射、吸收和自发荧光,导致其空间分辨率、成像灵敏度和对比度下降[18]。NIR-Ⅰ的波长不易被生物组织吸收和散射,因此在光学成像的应用中能够更深入有效地穿透皮肤、血液等生物组织[19-20]。同时,生物组织在此波段的自体荧光较小,SBR相对较高,是目前最适合用于动物的光穿透和深部组织成像的波段[21-22]。在2002年,美国Beth Israel Deaconess医学中心首次引进了一款具有独特特点的近红外荧光成像系统,该系统能够实时呈现彩色和近红外荧光图像,并且同时满足了获取近红外荧光信号和在手术视野中观察解剖结构这两个关键要求[23],这一具有突破性意义的技术提供了前所未有的便捷和精确性。基于上述优点,近红外荧光成像可以实现术中实时导航,显示未能直接暴露于表面的解剖结构,并在血管和输尿管等管腔器官以及肿瘤精准定位及识别得到了广泛的临床应用[24-25]。随着成像设备的更新升级,近红外荧光成像已经从独立波段成像发展到了双峰成像、多模态成像、3D荧光成像以及伪彩融合成像等多种成像模式[26-30],大幅度提高了医师在术中的视觉效果。 2.2 近红外荧光显影剂 在近红外波段内,生物体大多数组织很少产生近红外荧光,因此在近红外荧光成像的实际临床应用中,选择合适的显影剂至关重要。在过去的几十年里,研究人员在荧光引导外科手术中使用了荧光素、亚甲蓝以及5-氨基乙酰丙酸(5-aminolevulinic acid,5-ALA)等多种荧光染料进行探究。由于荧光素的发射波长与亚甲蓝类似于人体组织的自发性荧光,导致其作为近红外荧光显影剂的使用效果受到显影剂信号难以与人体自发性荧光区分的限制[31-32]。目前,5-ALA主要用于神经胶质瘤的手术中可视化治疗[33]。然而,这种药物在中国尚未获得临床批准。目前三碳花青系近红外荧光染料被证明是近红区理想的显影剂[34]。其中,吲哚菁绿是目前临床上应用最广泛的近红外荧光显影剂, 也是唯一经美国食品药品管理局(food and drug administration,FDA)和欧洲药品管理局(european medicines agency,EMA)批准的近红外造影剂[35]。吲哚菁绿的吸收峰和发射峰分别为805 nm和835 nm[36]。经静脉注射后,吲哚菁绿可以和血清蛋白迅速结合,经肝脏排出体外而在其他组织中不会被代谢掉,在血浆中半衰期为三四分钟[37]。在其引导下的近红外荧光成像已被用于多种疾病的检测和治疗,是目前在精准外科手术中应用比较成熟的术中成像方法,在术中肿瘤标记、血管造影、输尿管造影及前哨淋巴结识别以及胆管造影等方面得到了广泛的临床应用[38]。 2.3 近红外荧光成像在神经成像中的应用 术中神经荧光显影剂的应用可以实现神经荧光成像,特别是在由于外在暴力因素导致正常解剖位置发生位移或者破坏的情境下,能够明显降低神经识别的难度,提升手术的精准度。在近红外荧光的临床应用中,血管、输尿管和胆管等管状结构可以通过灌注荧光染料实现实时动态显像。相比之下,神经纤维的条束状结构使得普通染料很难着色,因此实现活体荧光成像难度很大。这也催生了科学家们的研究兴趣,一些神经荧光染料也在被探究。研究人员术前将荧光染料通过静脉注射或局部注射的方式注射到患者体内,使之与神经组织结合。术中,对目标神经区域照射近红外光,而后收集荧光染料所发出的荧光信号并传输到显示屏上。最后通过计算机软件根据需求,对收集到的荧光信号进行实时处理实现术中神经荧光成像,见图3。"
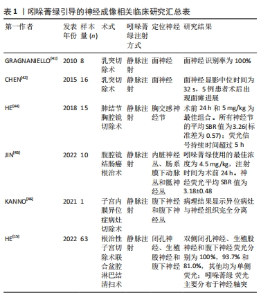
2.3.1 吲哚菁绿引导的神经成像 神经血管束是人体内的神经末梢与毛细血管缠绕在一起形成的微小解剖结构。将吲哚菁绿通过静脉注射到血液中,随着血液流动以及吲哚菁绿的渗透性和滞留增强,吲哚菁绿可以准确识别神经血管束来实现对神经的定位[39-40]。在2008年,GRAGNANIELLO等[41]使用新鲜的人体头部标本通过动脉注射吲哚菁绿在近红外荧光成像引导下实现了对面神经和半规管识别和保存。基于皮质骨和面神经的差异,在2014年,CHEN等[42]在乳突切除术中,骨化面神经管后,将标准剂量的吲哚菁绿在5 mL无菌水中稀释静脉注射到面神经管伴行血管实现了对面神经的可视化。这项技术的应用,帮助医师准确地找到面神经神经或者解剖标志,能够显著降低面神经损伤并发症的发生。然而,由于患者数量相对较少且部分为尸体验证,该技术并未形成一个明确的使用标准;此外,镜下荧光对面神经的可见性以及神经外科医师术中确定面神经位置具有主观性和不确定性;同时,该研究缺少对荧光图像的定量分析。在2016年,WENG等[43] 在应用胸腔镜吲哚菁绿显像检测肺转移性病变和纵隔占位病变时,发现胸交感神经节(包括星状神经节)可以在近红外模式下清晰显示。这是术中胸交感神经节荧光显像的首例报道,且患者治疗过程中未发现发生不良反应。随后,2018年该团队通过动物实验和临床实验对吲哚菁绿应用的最佳注射时间以及最佳剂量进行了探究[44]。在2019年,JIN等[45]在结肠癌根治术中,通过使用吲哚菁绿成功观察到内脏神经丛、肠系膜下动脉丛和骶神经丛。 在2020年,KANNO等[46]在治疗1例深度子宫内膜异位症的手术中,为了更好地显现病灶与盆腔自主神经的解剖关系,静脉注射吲哚菁绿进行近红外荧光成像,结果腹下神经和腹下神经丛在吲哚菁绿引导下明显突出,且术后并未发生盆腔自主神经损伤的相关不良反应,这是世界首例使用吲哚菁绿做神经保留的妇科手术。HE等[15]在2020年2月至2021年6月,开展了宫颈癌根治性子宫切除术中应用近红外荧光成像和吲哚菁绿识别盆腔神经的临床研究,评价了该项技术的可行性和安全性。这为术中识别盆腔神经、减少神经损伤及改善患者预后的提供了新思路,但由于该研究为单臂研究,没有设计随机对照试验来比较近红外荧光引导手术和传统手术的神经识别率和损伤率,因此该研究也存在一定的局限性。吲哚菁绿与不同组织的亲和力不同,与神经的亲和力较差,因此,实现神经的可视化需要增大吲哚菁绿的剂量。在实际临床应用吲哚菁绿存在量子产率低、光学稳定性差、不具有靶向性的缺点,但相比其他染料具有更好的组织穿透性,因此有人提出最理想的显影剂将是神经靶向物质与吲哚菁绿的混合物[47]。同时,在目前研究中,科研人员的兴趣更侧重于实际的临床应用,对于其吲哚菁绿与神经组织结合的具体机制还缺少研究佐证,见表1。"
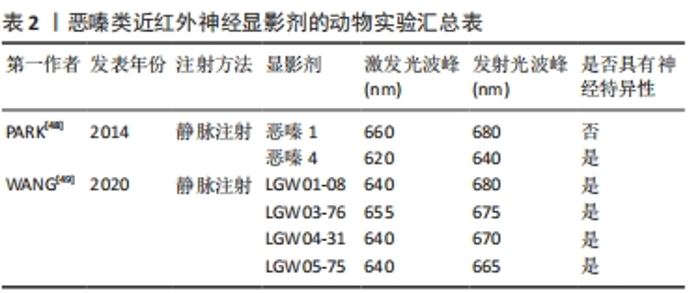
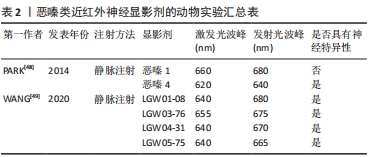
2.3.2 近红外靶向荧光团引导的神经成像 目前,具有神经特异性衍生物的恶嗪支架在发射光谱尾段恰好在近红外波段内,呈现出一种潜在的有前途和灵活的结构。恶嗪定向合成工程有望成为鉴定小分子神经造影剂候选物,实现在复杂的外科环境中有效地定位和识别神经[48-50]。PARK等[48]通过对具有近红外光谱的恶嗪类荧光团进行合成修饰,以恶嗪1和恶嗪4为支架,成功地建立了具有高度神经特异性荧光基团文库。WANG等[49]并通过体内神经荧光测试筛选了4种恶嗪衍生物(LGW01-08,LGW05-75,LGW04-31和LGW03-76)。与文库中其他基团相比,其中恶嗪4在神经特异性以及显影时间方面显示出较好的实验效果,成功地识别并实时突出了猪的喉返神经;而恶嗪1虽然与恶嗪4具有高度的结构相似性,但没有表现出神经特异性。部分研究成果见表2。"
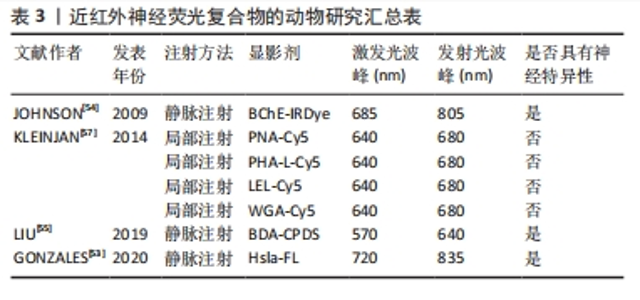
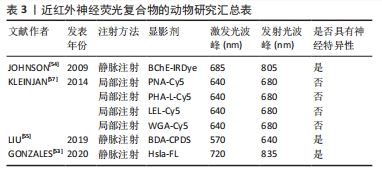
在2017年,有研究利用恶嗪4和尼罗红(脂质特异性恶嗪荧光团)不同的光谱特性,以局部给药的方式通过双色成像策略实现了神经和脂肪组织分离荧光[51]。在2019年,有研究继续通过对比恶嗪4与罗丹红在神经组织的荧光强度,探究了分配系数和总体电荷对于神经组织靶向和快速脱靶组织清除的影响[52]。尽管恶嗪在动物实验中显示出良好的效果,但是其荧光光谱并完全处于的近红外窗口内,因此其临床应用前景可能会受到限制。 2.3.3 近红外荧光团复合物引导的神经成像 大量神经标记结合位点的发现,使得手术时目标区域神经的体内荧光成像动态识别得到了极大的发展,这明显降低了手术中损伤神经的风险。 近年来,人们开始尝试将针对神经信号转到过程开发一些小分子物质和多肽物质人工复合物。例如,将作用于Na+门控通道的Hsla与Cy7.5染料合成的Hs1a-FL以及将作用于胆碱类物质的丁酰胆碱酯酶(BChE)使用近红外荧光染料标记后合成的BChE-IRDye均开始在动物实验中使用[53-54]。这些特殊分子能够作用于神经系统产生电活动的过程,从而与神经系统特异性结合。这给研究者们在未来神经特异性显影剂的开发提供了新的思路,研究者们可以将目标转向神经的电生理活动中。此外,研究人员将生物素化葡聚糖胺(BDA)与神经组织上的红色荧光碳化聚合物点(CPDS)相耦合,开发出BDA-CPDS,作为新一代的纳米神经荧光显影剂,其在体内体外均表现出良好的生物相容性和低毒性[55]。 蛋白聚糖是一类具有一个或多个附着聚糖链的蛋白质,是神经纤维结缔组织膜的主要蛋白[56]。研究证实,凝集素能够与周围神经组织上的蛋白聚糖亲和[56]。在2014年,KLEINJAN等[57]使用Cy5 结合小麦胚芽凝集素、花生凝集素、红芸豆凝集素及番茄凝集素4种凝集素通过局部注射的方法实现了小鼠坐骨神经神经荧光成像。由于凝集素只在神经外膜显示染色,其诱发全身毒性可能性较小,具有较大的临床应用潜力。由于凝集素只在神经外膜显示染色,其诱发全身毒性可能性较小,具有较大的临床应用潜力。当然,这些药物也有一些局限性。凝集素制剂只能采取局部注射的方式使用,因此很难实现深部神经组织的可视化。此外,由于这些凝集素剂能够与周围结缔组织产生相互作用,因此对成像效果会产生干扰,相关研究汇总见表3。"
| [1] SHI C, FLANAGAN SR, SAMADANI U. Vagus nerve stimulation to augment recovery from severe traumatic brain injury impeding consciousness: a prospective pilot clinical trial. Neurol Res. 2013;35(3):263-276. [2] CHAUDHARY IA, SAMIULLAH R, MASOOD R, et al. Recurrent laryngeal nerve injury: an experience with 310 thyroidectomies. J Ayub Med Coll Abbottabad. 2007;19(3):46-50. [3] MUSAT O, COLTA D, CERNAT C, et al. New perspectives in retinal imaging - angio OCT. Rom J Ophthalmol. 2016;60(2):63-67. [4] SENDEN RJ, DE JEAN P, MCAULEY KB, et al. Polymer gel dosimeters with reduced toxicity: a preliminary investigation of the NMR and optical dose-response using different monomers. Phys Med Biol. 2006;51(14):3301-3314. [5] CARL B, BOPP M, GJORGJEVSKI M, et al. Implementation of intraoperative computed tomography for deep brain stimulation: pitfalls and optimization of workflow, accuracy, and radiation exposure. World neurosurgery. 2018; S1878-8750(18)32902-92904. [6] FUCHS-BUDER T, SETTEMBRE N, SCHMARTZ D. Hybrid operating theater. Der Anaesthesist. 2018;67(7):480-487. [7] MARAVILLA K, BOWEN B. Imaging of the peripheral nervous system: evaluation of peripheral neuropathy and plexopathy. Am J Neuroradiol. 1998;19(6):1011-1023. [8] MISGELD T, KERSCHENSTEINER M. In vivo imaging of the diseased nervous system. Nature reviews Neuroscience. 2006;7(6):449-463. [9] CARVALHO C, SILVA-CORREIA J, OLIVEIRA J, et al. Nanotechnology in peripheral nerve repair and reconstruction. Adv Drug Deliv Rev. 2019;148: 308-343. [10] KIM SM, KIM SH, SEO DW, et al. Intraoperative neurophysiologic monitoring: basic principles and recent update. J Korean Med Sci. 2013; 28(9):1261-1269. [11] WALZ J, BURNETT A, COSTELLO A, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. European urology. 2010;57(2):179-192. [12] NGUYEN QT, TSIEN RY. Fluorescence-guided surgery with live molecular navigation--a new cutting edge. Nat Rev Cancer. 2013;13(9):653-662. [13] MIEOG JSD, ACHTERBERG FB, ZLITNI A, et al. Fundamentals and developments in fluorescence-guided cancer surgery. Nat Rev Clin Oncol. 2022;19(1):9-22. [14] CHI C, DU Y, YE J, et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics. 2014;4(11):1072-1084. [15] HE KS, LI PF, ZHANG ZY, et al. Intraoperative near-infrared fluorescence imaging can identify pelvic nerves in patients with cervical cancer in real time during radical hysterectomy. Eur J Nucl Med Mol Imaging. 2022;49(8): 2929-2937. [16] BEĆ KB, GRABSKA J, HUCK CW. Near-Infrared Spectroscopy in Bio-Applications. Molecules. 2020;25(12):2948. [17] VAN WILLIGEN DM, VAN DEN BERG NS, BUCKLE T, et al. Multispectral fluorescence guided surgery; a feasibility study in a phantom using a clinical-grade laparoscopic camera system. Am J Nucl Med Mol Imaging. 2017;7(3):138-147. [18] REN T, WANG Z, XIANG Z, et al. A General strategy for development of activatable nir-ii fluorescent probes for in vivo high-contrast bioimaging. Angew Chem Int Ed Engl. 2021;60(2):800-805. [19] BURGGRAAF J, KAMERLING I M, GORDON P B, et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med. 2015;21(8):955-961. [20] VAHRMEIJER A, HUTTEMAN M, VAN DER VORST J, et al. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013; 10(9):507-518. [21] DONNELLY P, FINE-GOULDEN M. How to use near-infrared spectroscopy. Arch Dis Child Educ Pract Ed. 2020;105(1):58-63. [22] SAKUDO A. Near-infrared spectroscopy for medical applications: current status and future perspectives. Clin Chim Acta. 2016;455:181-188. [23] BALACUMARASWAMI L, TAGGART D. Intraoperative imaging techniques to assess coronary artery bypass graft patency. Ann Thorac Surg. 2007; 83(6):2251-2257. [24] YANG Y, YANG X, LIU C, et al. Preliminary study on the application of en bloc resection combined with near-infrared molecular imaging technique in the diagnosis and treatment of bladder cancer. World J Urol. 2020;38(12):3169-3176. [25] YAN P, CHEN D, YAN X, et al. Ex vivo near-infrared molecular imaging of human upper urinary tract urothelial carcinoma with a CD47-based targeted tracer. Front Oncol. 2022;12:825476. [26] NTZIACHRISTOS V, BREMER C, WEISSLEDER R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13(1):195-208. [27] JATHOUL A, GROUNDS H, ANDERSON J, et al. A dual-color far-red to near-infrared firefly luciferin analogue designed for multiparametric bioluminescence imaging. Angew Chem Int Ed Engl. 2014;53(48):13059-13063. [28] WANG S, ZHAO Y, XU Y. Recent advances in applications of multimodal ultrasound-guided photoacoustic imaging technology. Vis Comput Ind Biomed Art. 2020;3(1):24. [29] CHOI J, TAAL A, MENG W, et al. Fully integrated time-gated 3D fluorescence imager for deep neural imaging. IEEE Trans Biomed Circuits Syst. 2020; 14(4):636-645. [30] HAO H, WANG X, QIN Y, et al. Ex vivo near-infrared targeted imaging of human bladder carcinoma by ICG-anti-CD47. Front Oncol. 2023;13:1083553. [31] OU C, LUO Y, HE L, et al. Application of fluorescence endoscopy with methylene blue dye and indocyanine green dual-tracer method in sentinel lymph node biopsy for women with breast cancer. Gland surgery. 2023; 12(6):780-790. [32] 胡跃东,张梦梦,张俊文,等.荧光素酶标记胶质瘤细胞模型的构建及其在溶瘤病毒治疗中的应用[J].中华神经外科杂志,2022,38(8):843-848. [33] MARAGKOS G, SCHüPPER A, LAKOMKIN N, et al. Fluorescence-guided high-grade glioma surgery more than four hours after 5-aminolevulinic acid administration. Front Neurol. 2021;12:644804. [34] DU Y, LIU X, ZHU S. Near-infrared-II cyanine/polymethine dyes, current state and perspective. Front Chem. 2021;9:718709. [35] GIRAUDEAU C, MOUSSARON A, STALLIVIERI A, et al. Indocyanine green: photosensitizer or chromophore? Still a debate. Curr Med Chem. 2014. doi:10.2174/0929867321666131218095802. [36] 王晓颖.吲哚菁绿荧光成像技术在肝脏外科应用中国专家共识(2023版)[J].中国实用外科杂志,2023,43(4):371-383. [37] REINHART M, HUNTINGTON C, BLAIR L, et al. Indocyanine green:historical context, current applications, and future considerations. Surg Innov. 2016; 23(2):166-175. [38] CHEN Y, ZHANG H, LEI Z, et al. Recent advances in intraoperative nerve bioimaging: fluorescence‐guided surgery for nerve preservation. Small Struct. 2020. doi:10.1002/sstr.202000036. [39] MANGANO MS, DE GOBBI A, BENIAMIN F, et al. Robot-assisted nerve-sparing radical prostatectomy using near-infrared fluorescence technology and indocyanine green:initial experience. Urologia. 2018;85(1):29-31. [40] JIANG JX, KEATING JJ, JESUS EM, et al. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Mol Imaging. 2015;5(4):390-400. [41] GRAGNANIELLO C, KAMEL M, AL-MEFTY O. Utilization of fluorescein for identification and preservation of the facial nerve and semicircular canals for safe mastoidectomy: a proof of concept laboratory cadaveric study. Neurosurgery. 2010;66(1):204-207. [42] CHEN SC, WANG MC, WANG WH, et al. Fluorescence-assisted visualization of facial nerve during mastoidectomy: a novel technique for preventing iatrogenic facial paralysis. Auris Nasus Larynx. 2015;42(2):113-118. [43] WENG W, LIU Y, ZHOU J, et al. Thoracoscopic indocyanine green near-infrared fluorescence for thoracic sympathetic ganglions. Ann Thorac Surg. 2016;101(6):2394. [44] HE KS, ZHOU J, YANG F, et al. Near-infrared intraoperative imaging of thoracic sympathetic nerves: from preclinical study to clinical trial. Theranostics. 2018;8(2):304-313. [45] JIN H, ZHENG L, LU L, et al. Near-infrared intraoperative imaging of pelvic autonomic nerves: a pilot study. Surg Endosc. 2022;36(4):2349-2356. [46] KANNO K, AIKO K, YANAI S, et al. Clinical use of indocyanine green during nerve-sparing surgery for deep endometriosis. Fertil Steril. 2021;116(1): 269-271. [47] HANDGRAAF HJ, VERBEEK FP, TUMMERS QR, et al. Real-time near-infrared fluorescence guided surgery in gynecologic oncology: a review of the current state of the art. Gynecol Oncol. 2014;135(3):606-613. [48] PARK MH, HYUN H, ASHITATE Y, et al. Prototype nerve-specific near-infrared fluorophores. Theranostics. 2014;4(8):823-833. [49] WANG LG, BARTH CW, KITTS CH, et al. Near-infrared nerve-binding fluorophores for buried nerve tissue imaging. Sci Transl Med. 2020;12(542): eaay0712. [50] BARTH CW, GIBBS SL. Visualizing Oxazine 4 nerve-specific fluorescence ex vivo in frozen tissue sections. Proc SPIE Int Soc Opt Eng. 2016;9696:96960R. [51] BARTH CW, GIBBS SL. Direct administration of nerve-specific contrast to improve nerve sparing radical prostatectomy. Theranostics. 2017;7(3):573-593. [52] WANG LG, BARTH CW, COMBS JR, et al. Investigation of oxazine and rhodamine derivatives as peripheral nerve tissue targeting contrast agent for in vivo fluorescence imaging. Proc SPIE Int Soc Opt Eng. 2019; 10862:108620H. [53] GONZALES J, PIROVANO G, CHOW CY, et al. Fluorescence labeling of a NaV1.7-targeted peptide for near-infrared nerve visualization. EJNMMI Res. 2020;10(1):49. [54] JOHNSON N D, DUYSEN EG, LOCKRIDGEO. Intrathecal delivery of fluorescent labeled butyrylcholinesterase to the brains of butyrylcholinesterase knock-out mice: visualization and quantification of enzyme distribution in the brain. Neurotoxicology. 2009;30(3):386-392. [55] LIU Y, LIU J, ZHANG J, et al. A brand-new generation of fluorescent nano-neural tracers: biotinylated dextran amine conjugated carbonized polymer dots. Biomater Sci. 2019;7(4):1574-1583. [56] DYCK SM, KARIMI-ABDOLREZAEE S. Chondroitin sulfate proteoglycans: key modulators in the developing and pathologic central nervous system. Exp Neurol. 2015;269:169-187. [57] KLEINJAN GH, BUCKLE T, VAN WILLIGEN DM, et al. Fluorescent lectins for local in vivo visualization of peripheral nerves. Molecules. 2014;19(7):9876-9892. [58] LI B, ZHAO M, LIN J, et al. Management of fluorescent organic/inorganic nanohybrids for biomedical applications in the NIR-II region. Chem Soc Rev. 2022;51(18):7692-7714. [59] ZHAO M, WANG J, LEI Z, et al. NIR-II pH sensor with a FRET adjustable transition point for in situ dynamic tumor microenvironment visualization. Angew Chem Int Ed Engl. 2021;60(10):5091-5095. [60] LI B, LIU H, HE Y, et al. A “Self-Checking” pH/Viscosity-activatable NIR-II molecule for real-time evaluation of photothermal therapy efficacy. Angew Chem Int Ed Engl. 2022;61(16):e202200025. [61] CAO Z, FENG L, ZHANG G, et al. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials. 2018; 155:103-111. [62] FENG L, WU L, QU X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv Mater. 2013;25(2):168-186. [63] PAJOUHESH H, LENZ GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2(4):541-553. [64] FAGERHOLM U. The highly permeable blood-brain barrier: an evaluation of current opinions about brain uptake capacity. Drug Discov Today. 2007; 12(23-24):1076-1082. [65] WALSH EM, COLE D, TIPIRNENI KE, et al. Fluorescence imaging of nerves during surgery. Ann Surg. 2019;270(1):69-76. [66] WATERHOUSE RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5(6):376-389. [67] SAJI H. In vivo molecular imaging. Biol Pharm Bull. 2017;40(10):1605-1615. [68] GIBBS-STRAUSS SL, NASR KA, FISH KM, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Molecular Imaging. 2011;10(2): 91-101. [69] GIBBS SL, XIE Y, GOODWILL HL, et al. Structure-activity relationship of nerve-highlighting fluorophores. PLoS One. 2013;8(9):e73493. |
| [1] | Wu Tong, Yin Caiyun, Zhao Mingzhe, Zhu Yishen. Application of functional peptides for biomedical diagnosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 478-485. |
| [2] | Fan Yaru, Li Ruixin, Li Fengji, Luo Rui, Liu Hao, Yan Yingbin. Characterization and photothermal effect of indocyanine green encapsulated poly lactic acid-co-glycolic acid microspheres [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1817-1823. |
| [3] | FAN Yaru, LI Ruixin , LI Fengji, LUO Rui, LIU Hao, YAN Yingbin. Characterization and photothermal effect of indocyanine green encapsulated poly lactic acid-co-glycolic acid microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [4] | Li Xuan, Lu Min, Li Mingxing, Ao Meng, Tang Linmei, Zeng Zhen, Hu Jingwei, Huang Zhiqiang, Xuan Jiqing. In vitro multi-modal imaging of magnetic targeted nanoparticles and their targeting effect on hepatic stellate cells [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 566-571. |
| [5] | Hong Xia, Shi Xiao-tian, Wang Kun-ju, Lin Lin-ran. Three-dimensional reconstruction of pelvic arteries by laminagraphy of pipe cast specimens [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(9): 1660-1664. |
| [6] | 徐泽鹏,马腾飞,李凯扬. Insight into the three-wavelength hepatic reserve function detection system [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(5): 902-906. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||