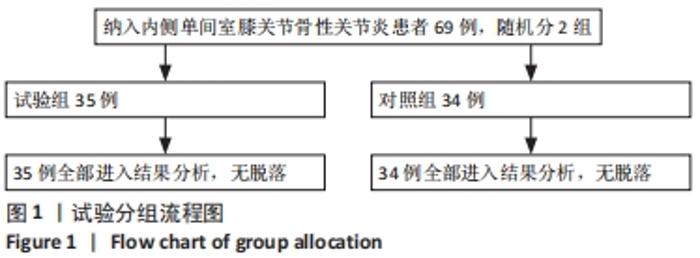
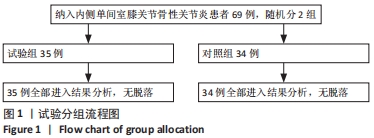
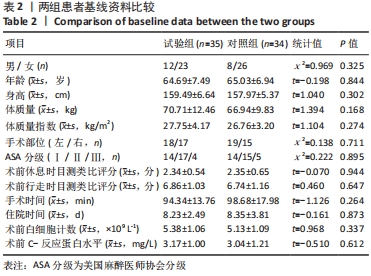
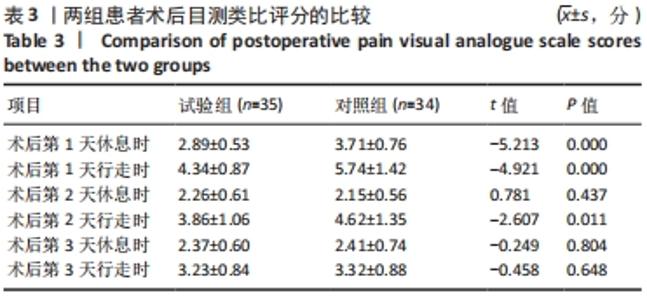
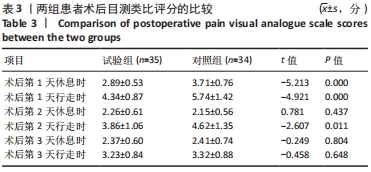
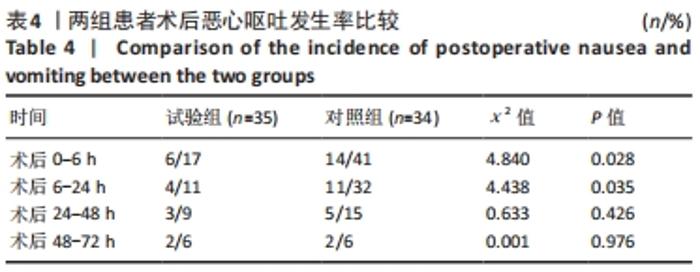
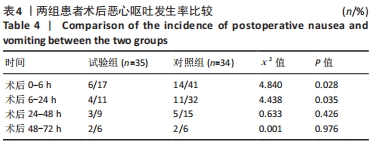
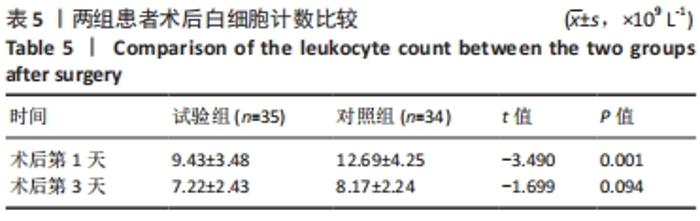
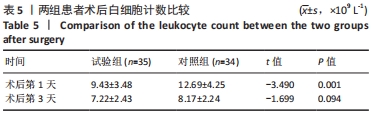
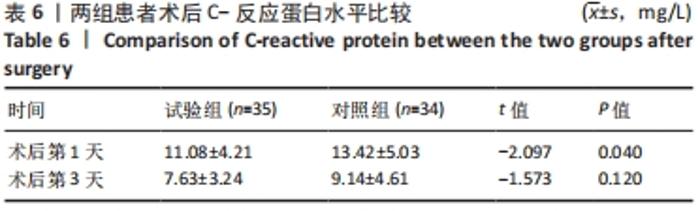
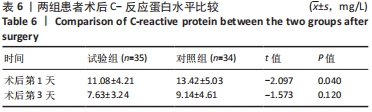
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (33): 5297-5302.doi: 10.12307/2022.749
Previous Articles Next Articles
Effect of intravenous dexamethasone preoperatively on pain and complications after unicondylar arthroplasty
Wang Zhenheng, Wang Zhidong, Liu Naicheng, Chen Guangdong, Gao Maofeng, Shi Weidong, Zhu Ruofu
- Department of Orthopedics, The First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2021-12-04Accepted:2021-12-31Online:2022-11-28Published:2022-03-30 -
Contact:Zhu Ruofu, MD, Chief physician, Professor, Department of Orthopedics, The First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Wang Zhenheng, Attending physician, MD, Department of Orthopedics, The First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:the Youth Science Fund Project of National Natural Science Foundation of China, No. 81802223 (to WZH)
CLC Number:
Cite this article
Wang Zhenheng, Wang Zhidong, Liu Naicheng, Chen Guangdong, Gao Maofeng, Shi Weidong, Zhu Ruofu. Effect of intravenous dexamethasone preoperatively on pain and complications after unicondylar arthroplasty[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(33): 5297-5302.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] KLUG A, GRAMLICH Y, RUDERT M, et al. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future heath care systems over the next 30 years. Knee Surg. Sports Traumatol. Knee Surg Sports Traumatol Arthrosc. 2021; 29(10):3287-3298. [2] BORUS T, THORNHILL T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg. 2008;16(1):9-18. [3] MURRAY DW, PARKINSON RW. Usage of unicompartmental knee arthroplasty. Bone Joint J. 2018;100B(4):432-435. [4] FORNELL S, PRADA E, BARRENA P, et al. Mid-term outcomes of mobile-bearing lateral unicompartmental knee arthroplasty. Knee. 2018;25(6): 1206-1213. [5] POLAT AE, POLAT B, GURPINAR T, et al. Factors affecting the functional outcome of oxford phase 3 unicompartmental knee arthroplasty. Acta Ortop Bras. 2020;28(2):78-83. [6] KORT NP, BEMELMANS YFL, SCHOTANUS MGM. Outpatient surgery for unicompartmental knee arthroplasty is effective and safe. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2659-2667. [7] MATSUMOTO M, SAITO S, ANDREWS S, et al. Barriers to achieving same day discharge following unilateral unicompartmental knee arthroplasty. Knee. 2020;27(5): 1365-1369. [8] TAMMACHOTE N, KANITNATE S. Intravenous Dexamethasone Injection Reduces Pain From 12 to 21 Hours After Total Knee Arthroplasty: A Double-Blind, Randomized, Placebo-Controlled Trial. J Arthroplast. 2020;35(2):394-400. [9] KELLY ME, TURCOTTE JJ, AJA JM, et al. Impact of Dexamethasone on Length of Stay and Early Pain Control in Direct Anterior Approach Total Hip Arthroplasty With Neuraxial Anesthesia. J Arthroplast. 2021;36(3): 1009-1012. [10] LI DH, WANG QR, ZHAO X, et al. Comparison of Intravenous and Topical Dexamethasone for Total Knee Arthroplasty: A Randomized Double-Blinded Controlled Study of Effects on Dexamethasone Administration Route and Enhanced Recovery. J Arthroplast. 2021;36(5):1599-1606. [11] GAN TJ, BELANI KG, BERGESE S, et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth Analg. 2020;131(2):411-448. [12] LI XF, XU G, XIE WJ, et al. The efficacy and safety of dexamethasone for pain management after total knee arthroplasty: A systematic review and meta-analysis. Int J Surg. 2018;53:65-71. [13] FAN ZR, MA JX, MA XL, et al. The efficacy of dexamethasone on pain and recovery after total hip arthroplasty: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97(13):e0100. [14] RYTTER S, STILLING M, MUNK S, et al. Methylprednisolone reduces pain and decreases knee swelling in the first 24 h after fast-track unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):284-290. [15] 田海涛,王远贺,田少奇,等.膝关节置换应用甲基泼尼松龙对术后恶心呕吐和疼痛的影响及安全性评估[J].中国组织工程研究, 2017,21(3):335-339. [16] XU H, ZHANG SY, XIE JW, et al. Multiple Doses of Perioperative Dexamethasone Further Improve Clinical Outcomes After Total Knee Arthroplasty: A Prospective, Randomized, Controlled Study. J Arthroplast. 2018;33(11):3448-3454. [17] BREKKE AC, AMARO EJ, POSEY SL, et al. Do Corticosteroids Attenuate the Peri-Operative Acute Phase Response After Total Knee Arthroplasty? J Arthroplast. 2019;34(1):27-35. [18] BACKSTEIN D. In TKA, Preoperative Systemic Steroids Reduced Pain and Nausea at 24 Hours After Surgery; Postoperative Administration Reduced Pain and Nausea at 48 Hours. J Bone Joint Surg Am. 2020; 102(10):908-908. [19] KIM JK, RO DH, LEE HJ, et al. Efficacy of Systemic Steroid Use Given One Day After Total Knee Arthroplasty for Pain and Nausea: A Randomized Controlled Study. J Arthroplast. 2020;35(1):69-75. [20] MOCHIZUKI T, SATO T, BLAHA JD, et al. Kinematics of the knee after unicompartmental arthroplasty is not the same as normal and is similar to the kinematics of the knee with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1911-1917. [21] TILLE E, BEYER F, AUERBACH K, et al. Better short-term function after unicompartmental compared to total knee arthroplasty. BMC Musculoskelet Disord. 2021;22(1):326. [22] MUNK S, DALSGAARD J, BJERGGAARD K, et al. Early recovery after fast-track Oxford unicompartmental knee arthroplasty 35 patients with minimal invasive surgery. Acta Orthop. 2012;83(1):41-45. [23] LAKRA A, MURTAUGH T, SHAH RP, et al. Early Postoperative Pain Predicts 2-Year Functional Outcomes following Knee Arthroplasty. J Knee Surg. 2020;33(11):1132-1139. [24] LYGRE SHL, ESPEHAUG B, HAVELIN LI, et al. Pain and Function in Patients After Primary Unicompartmental and Total Knee Arthroplasty. J Bone Joint Surg Am. 2010;92A(18):2890-2897. [25] GAN TJ, MEYER T, APFEL CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97(1):62-71. [26] PHILLIPS C, BROOKES CD, RICH J, et al. Postoperative nausea and vomiting following orthognathic surgery. Int J Oral Maxillofac Surg. 2015;44(6):745-751. [27] BERGER RA, KUSUMA SK, SANDERS SA, et al. The Feasibility and Perioperative Complications of Outpatient Knee Arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449. [28] RUIZ N, BUISSON X, FILIPPI G, et al. Ambulatory unicompartmental knee arthroplasty: Short outcome of 50 first cases. Orthop Traumatol Surg Res. 2018;104(7):961-966. [29] SAKKA BI, SHIINOKI A, MORIKAWA L, et al. Comparison of early post-operative complications following unilateral or single-stage bilateral unicompartmental knee arthroplasty. Knee. 2020;27(5):1406-1410. [30] CHU CC, HSING CH, SHIEH JP, et al. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol. 2014;72:248-254. [31] ROMUNDSTAD L, STUBHAUG A. Glucocorticoids for acute and persistent postoperative neuropathic pain - What is the evidence? Anesthesiology. 2007;107(3):371-373. [32] ZHOU GH, MA LP, JING JH, et al. A meta-analysis of dexamethasone for pain management in patients with total knee arthroplasty. Medicine (Baltimore). 2018;97(35):e11753. [33] FAN ZR, MA JX, KUANG MJ, et al. The efficacy of dexamethasone reducing postoperative pain and emesis after total knee arthroplasty: A systematic review and meta-analysis. Int J Surg. 2018;52:149-155. [34] LEI Y, HUANG Z, HUANG Q, et al. Is a split-dose intravenous dexamethasone regimen superior to a single high dose in reducing pain and improving function after total hip arthroplasty? A randomized blinded placebo-controlled trial. Bone Joint J. 2020;102B(11):1497-1504. [35] NG YCS, LO NN, YANG KY, et al. Effects of periarticular steroid injection on knee function and the inflammatory response following unicondylar knee arthroplasty. Knee Surg Sport Tr A. 2011;19(1):60-65. [36] PANG HN, LO NN, YANG KY, et al. Peri-articular steroid injection improves the outcome after unicondylar knee replacement - A prospective, randomised controlled trial with a two-year follow-up. J Bone Joint Surg Br. 2008;90B(6):738-744. [37] LUCERO CM, GARCIA-MANSILLA A, ZANOTTI G, et al. A Repeat Dose of Perioperative Dexamethasone Can Effectively Reduce Pain, Opioid Requirement, Time to Ambulation, and In-Hospital Stay After Total Hip Arthroplasty: A Prospective Randomized Controlled Trial. J Arthroplast. 2021;36(12):3938-3944. [38] NIELSEN NI, KEHLET H, GROMOV K, et al. High-dose steroids in high pain responders undergoing total knee arthroplasty: a randomised double-blind trial. Br J Anaesth. 2022;128(1):150-158. [39] ZHUO Y, YU R, WU C, et al. The role of perioperative intravenous low-dose dexamethasone in rapid recovery after total knee arthroplasty: a meta-analysis. J Int Med Res. 2021;49(3):300060521998220. [40] WU Y, LU X, MA Y, et al. Perioperative multiple low-dose Dexamethasones improves postoperative clinical outcomes after Total knee arthroplasty. BMC Musculoskelet Disord. 2018;19(1):428. [41] DE OLIVEIRA GS, ALMEIDA MD, BENZON HT, et al. Perioperative Single Dose Systemic Dexamethasone for Postoperative Pain A Meta-analysis of Randomized Controlled Trials. Anesthesiology. 2011;115(3):575-588. |
| [1] | Li Jiajun, Xia Tian, Liu Jiamin, Chen Feng, Chen Haote, Zhuo Yinghong, Wu Weifeng. Molecular mechanism by which icariin regulates osteogenic signaling pathways in the treatment of steroid-induced avascular necrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 780-785. |
| [2] | Song Wei, Zhang Yaxin, Jia Dazhou, Sun Yu. Adjective application of dexamethasone combined with furosemide for early pain and swelling after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(33): 5317-5322. |
| [3] | Song Jianzhi, Xu Lisen, Zhang Chen, Tu Feng, Niu Fei. Mechanism by which SC79, an Akt activator, inhibits dexamethasone-induced apoptosis and programmed necrosis of osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(29): 4699-4703. |
| [4] | Luo Qiurui, Song Xiaoqin, Wang Rongli. Effects of sirtinol versus dexamethasone on the levels of recombinant sirtuin 1 and interleukin-5 in asthmatic mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2720-2725. |
| [5] | Liu Fatai, Yang Jinshun, Zhong Weibin. Application of three-dimensional printed osteotomy guide plate in knee arthroplasty in osteoarthritis patients with concomitant femoral deformity [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(15): 2312-2316. |
| [6] | Liu Zhiqiang, Liang Yuyao, Gao Ge, Meng Shun, Xie Zhongqi, Su Yu. Influence of surface treatment technology on friction and wear performance of artificial joints [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1604-1609. |
| [7] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [8] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [9] | Li Shibin, Lai Yu, Zhou Yi, Liao Jianzhao, Zhang Xiaoyun, Zhang Xuan. Pathogenesis of hormonal osteonecrosis of the femoral head and the target effect of related signaling pathways [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 935-941. |
| [10] | Li Yan, Wang Pei, Deng Donghuan, Yan Wei, Li Lei, Jiang Hongjiang. Electroacupuncture for pain control after total knee arthroplasty: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 957-963. |
| [11] | Wang Weigang, Yang Zhidong, Feng Zongquan, Wang Ding. A mid-term clinical follow-up of unicompartmental knee arthroplasty with fixed bearing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 368-373. |
| [12] | He Shengcai, Jiang Chengdan, Jing Chunxiang, Luo Minyi, Ma Xingfa, Li Jiazhou, Pan Huashan. Values of alpha defensin and C-reactive protein in synovial fluid in the diagnosis of periprosthetic infection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(27): 4344-4347. |
| [13] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [14] | Xu Hui, Kang Bingxin, Gao Chenxin, Zhao Chi, Xu Xirui, Sun Songtao, Xie Jun, Xiao Lianbo, Shi Qi. Effectiveness of Tuina in the treatment of pain after total knee arthroplasty in patients with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(18): 2840-2845. |
| [15] | Gao Zhixiang, Xiao Cong, Yang Hongtao, Meng Xiangyu. Statistical decision tree model analysis on hidden blood loss in the perioperative period of thoracolumbar burst fracture accompanied with neurological deficiency [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(15): 2364-2369. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||