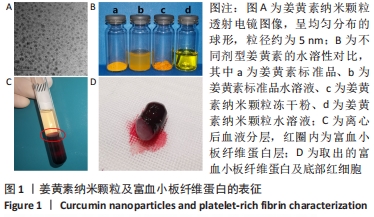
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (27): 4300-4307.doi: 10.12307/2022.859
Previous Articles Next Articles
Platelet-rich fibrin combined with curcumin nanoparticle hydrogel promotes wound healing in diabetic mice
Wu Haineng1, Geng Kang1, Wang Jing2, Xiong Aibing1
- 1Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, National Clinical Key Construction Specialty, Luzhou 646000, Sichuan Province, China; 2Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Received:2021-10-14Accepted:2021-12-10Online:2022-09-28Published:2022-03-12 -
Contact:Xiong Aibing, Master, Chief physician, Professor, Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, National Clinical Key Construction Specialty, Luzhou 646000, Sichuan Province, China -
About author:Wu Haineng, Master candidate, Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, National Clinical Key Construction Specialty, Luzhou 646000, Sichuan Province, China -
Supported by:Natural Science Youth Fund Project of Southwest Medical University, No. 2020ZRQNA008 (to GK)
CLC Number:
Cite this article
Wu Haineng, Geng Kang, Wang Jing, Xiong Aibing. Platelet-rich fibrin combined with curcumin nanoparticle hydrogel promotes wound healing in diabetic mice[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4300-4307.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
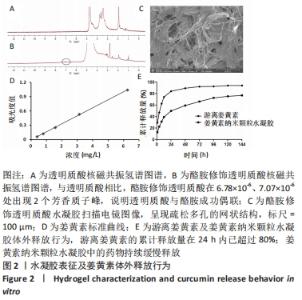
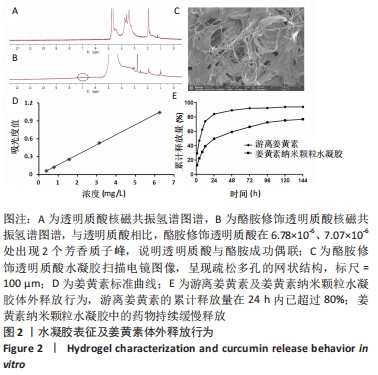
核磁共振氢谱检测结果显示,与透明质酸相比,酪胺修饰透明质酸在6.78×10-6、7.07×10-6处出现2个芳香质子峰,说明透明质酸与酪胺成功偶联,见图2A,B。扫描电镜结果表明,酪胺修饰透明质酸水凝胶呈现疏松多孔的网状结构,该结构有利于药物的吸附和缓释,见图2C。 2.2 姜黄素纳米颗粒水凝胶的姜黄素体外释放行为 通过紫外分光光度计测定不同浓度下的姜黄素吸光度,其标准曲线为:y=0.168x-0.003 8,r2=0.999 7,表明其线性相关关系良好,见图2D。由此测得10%姜黄素纳米颗粒实际载药率为(9.26±0.02)%,包封率为(92.63±0.15)%。 体外释放实验结果表明,游离姜黄素和姜黄素纳米颗粒水凝胶的释放曲线均呈现先陡峭后平缓的趋势。游离姜黄素组的累计释放量在24 h内已超过80%,此后的药物释放无明显增加;然而姜黄素纳米颗粒水凝胶组中的药物持续缓慢释放,有利于药物维持更长效的作用,见图2E。"
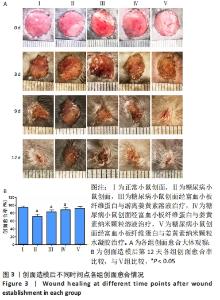
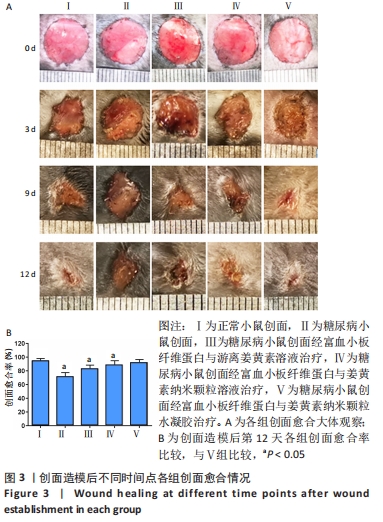
2.3 各组糖尿病造模一般情况 A组小鼠毛色光亮,精神良好,行为活跃。B组小鼠在注射链脲佐菌素后逐渐出现多饮、多食、多尿、毛发粗糙萎黄、精神倦怠、活动减少等表现,并且体质量较A组逐渐减轻,体型消瘦。 2.4 各组小鼠体质量及血糖变化 普通维持饲料适应性喂养1周后,A组与B组小鼠体质量比较差异无显著性意义(P > 0.05)。B组小鼠给予高脂高糖饲料喂养8周后,体质量比A组小鼠稍重。A组小鼠注射生理盐水1周后体质量较前稍有增长,B组小鼠注射链脲佐菌素1周后质量较前明显降低。 普通维持饲料适应性喂养1周后,A组与B组小鼠血糖值比较差异无显著性意义(P > 0.05)。B组小鼠给予高脂高糖饲料喂养8周后,其血糖值较A组高(P < 0.05)。注射链脲佐菌素1周后,B组小鼠血糖值较A组明显升高(P < 0.01),且大于16.7 mmol/L,说明2型糖尿病造模成功。 2.5 各组小鼠创面愈合情况 观察期间各组小鼠创面逐渐愈合,表面覆盖大小不等的痂壳,痂下间隙及创面周围有不同程度红肿、渗出,均无感染、化脓现象。创面造模后第3天,各组创面面积均有不同程度减小,表面均有肉芽组织生长,但B组创面愈合面积不及其他各组。创面造模后第9天,各组创面面积进一步缩小,A、C、D、E组创面面积显著减小,肉芽组织逐渐纤维化,质地较韧。在各药物干预组中,以E组创面愈合情况最佳。B组创面表面仍以痂壳覆盖,肉芽组织成熟缓慢。创面造模后第12天,A、C、D、E组创面基本愈合完全,B组创面面积较前虽有明显缩小,但仍有部分创面未愈合,见图3A。 创面造模后第12天,A-E组创面愈合率分别为(94.39±3.60)%,(71.17±6.32)%,(82.78±5.58)%,(88.33±6.37)%,(92.17±4.27)%。单因素方差分析显示,5组创面愈合率比较差异有显著性意义(F=54.182,P < 0.001)。LSD-t法两两比较显示,E组创面愈合率高于B、C、D组(P均< 0.05),与A组比较差异无显著性意义(P=0.216),见图3B。"
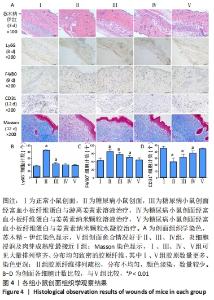
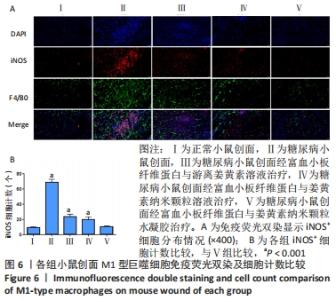
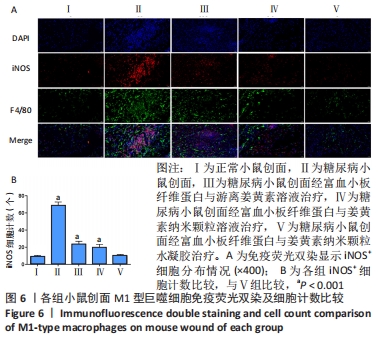
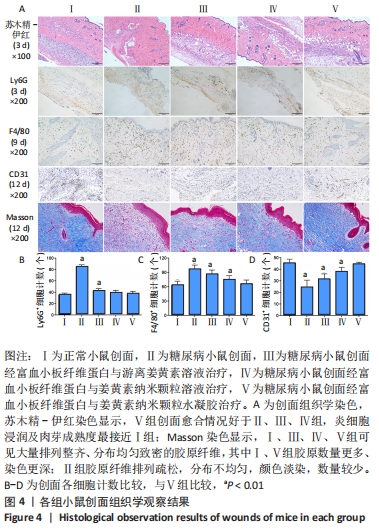
2.6 各组小鼠创面组织形态学观察 创面造模后第3天的苏木精-伊红染色结果显示,A组创面大量炎症细胞浸润,可见丰富的新生毛细血管及成纤维细胞分布;B组创面炎症细胞稀疏,可见少量散在孤立的毛细血管;C组创面可见一定数量的炎症细胞、毛细血管及成纤维细胞;D组创面可见较多的炎症细胞、新生毛细血管和成纤维细胞;E组创面炎症细胞浸润明显,伴有大量的新生毛细血管和成纤维细胞,见图4A。 2.7 各组小鼠创面中性粒细胞及巨噬细胞比较 创面造模后第3天,各组小鼠创面Ly6G+细胞分布情况,见图4A。A-E组创面Ly6G+细胞平均数量分别为(34.80±3.78),(84.13±4.31),(41.67±4.65),(38.37±4.54),(36.30±5.42)个。各组Ly6G+细胞平均数量行方差齐性检验(P=0.349),单因素方差分析结果显示,5组间Ly6G+细胞平均数量比较差异有显著性意义(F=626.322,P<0.001)。LSD-t法两两比较结果显示,E组Ly6G+细胞平均数量低于B、C组(P均< 0.001),与A、D组比较差异无显著性意义(P=0.206,0.082),见图4B。 创面造模后第9天,各组小鼠创面F4/80+细胞分布情况,见图4A。A-E组创面F4/80+细胞平均数量分别为(62.17±9.48),(95.60±9.61),(85.20±9.66),(73.60±9.41),(64.47±9.46)个。各组F4/80+细胞平均数量行方差齐性检验(P=0.913),单因素方差分析结果显示,5组间F4/80+细胞平均数量比较差异有显著性意义(F=66.020,P < 0.001)。LSD-t法两两比较结果显示,E组F4/80+细胞平均数量低于B、C、D组(P均< 0.001),与A组比较差异无显著性意义(P=0.351),见图4C。"
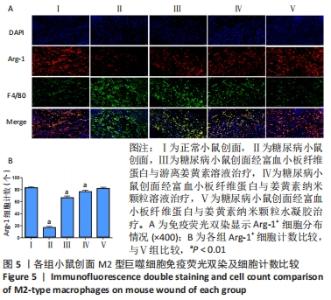
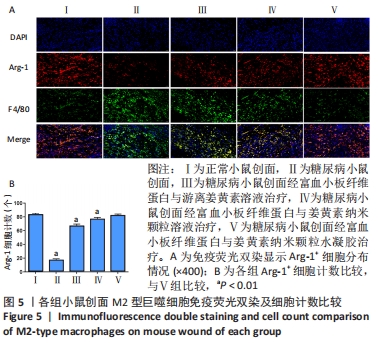
2.8 各组小鼠创面巨噬细胞极化分型 创面造模后第9天,各组小鼠创面M2型巨噬细胞免疫荧光双染结果,见图5A。A-E组创面Arg-1阳性细胞平均数量分别为(83.20±2.17),(16.60±2.41),(66.80±2.77),(76.80±2.39),(82.00±2.12)个。各组Arg-1阳性细胞平均数量行方差齐性检验(P=0.920),单因素方差分析显示,5组间Arg-1阳性细胞平均数量比较差异有显著性意义(F=683.373,P < 0.001)。LSD-t法两两比较结果显示,E组Arg-1阳性细胞平均数高于B、C、D组(P均< 0.01),与A组比较差异无显著性意义(P=0.435),见图5B。"

2.9 各组小鼠创面胶原纤维形成情况 创面造模第12天的Masson染色结果显示(蓝色为胶原分布区域),A、C、D、E组可见大量排列整齐、分布均匀致密的胶原纤维,其中A组和E组胶原数量更多、染色更深;B组虽然也可见胶原纤维分布,但排列疏松、分布不均匀、颜色淡染,数量也不及其他各组,见图4A。 2.10 各组小鼠创面血管生成情况 创面造模第12天,各组小鼠创面CD31+细胞分布情况,见图4A。A-E组创面CD31+细胞平均数量分别为(44.90±3.81),(23.80±6.80),(30.90±5.09),(37.50±4.22),(43.90±2.18)个。各组CD31+细胞平均数量行方差齐性检验(P=0.229),单因素方差分析显示,5组间CD31+细胞平均数量比较差异有显著性意义(F=36.461,P < 0.001)。LSD-t法两两比较结果显示,E组CD31+细胞平均数量高于B、C、D组(P均< 0.01),与A组比较差异无显著性意义(P=0.635),见图4D。 2.11 材料生物相容性 由动物创面组织学观察结果可知,富血小板纤维蛋白与姜黄素纳米颗粒水凝胶具有良好的组织相容性。"

| [1] ZHANG P, LU J, JING Y, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017; 49(2):106-116. [2] RAMIREZ-ACUÑA JM, CARDENAS-CADENA SA, MARQUEZ-SALAS PA, et al. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics (Basel). 2019;8(4):193. [3] HARDING JL, PAVKOV ME, GREGG EW, et al. Trends of Nontraumatic Lower-Extremity Amputation in End-Stage Renal Disease and Diabetes: United States, 2000-2015. Diabetes Care. 2019;42(8):1430-1435. [4] 冉兴无,杨兵全,许樟荣.我国糖尿病足病的诊治现状与未来的研究方向[J].中华糖尿病杂志,2014,6(7):437-439. [5] SKREPNEK GH, MILLS JL SR, LAVERY LA, et al. Health Care Service and Outcomes Among an Estimated 6.7 Million Ambulatory Care Diabetic Foot Cases in the U.S. Diabetes Care. 2017;40(7):936-942. [6] 姜玉峰.中国体表慢性难愈合创面流行病学研究[D].北京:中国人民解放军军医进修学院,2011. [7] 李炳旻,王芳芳,李倩坤,等.糖尿病溃疡的发生机制与治疗进展[J].解放军医学院学报,2019,40(10):992-994. [8] 姜臻宇.糖尿病足患者流行病学调查及其截肢的相关危险因素分析[D].南昌:南昌大学,2020. [9] CHIARAVALLOTI AJ, ZUBKOV B, ZUBKOV A. Treatment of a Chronic Cutaneous Surgical Wound With Platelet-Rich Fibrin. Dermatol Surg. 2018;44(3):449-452. [10] KARIMI K, ROCKWELL H. The Benefits of Platelet-Rich Fibrin. Facial Plast Surg Clin North Am. 2019;27(3):331-340. [11] SOUSA F, MACHADO V, BOTELHO J, et al. Effect of A-PRF Application on Palatal Wound Healing after Free Gingival Graft Harvesting: A Prospective Randomized Study. Eur J Dent. 2020;14(1):63-69. [12] LI Y, ZHAO S, DER LV, et al. Efficacy of curcumin for wound repair in diabetic rats/mice: a systematic review and meta-analysis of preclinical studies. Curr Pharm Des. 2022;28(3):187-197. [13] DENG X, LI F, LI Y, et al. Effect of curcumin on wound healing in a murine model of diabetic foot. J Biol Regul Homeost Agents. 2020;34(5):1879-1884. [14] GHUFRAN H, MEHMOOD A, AZAM M, et al. Curcumin preconditioned human adipose derived stem cells co-transplanted with platelet rich plasma improve wound healing in diabetic rats. Life Sci. 2020;257: 118091. [15] NOWAK NC, MENICHELLA DM, MILLER R, et al. Cutaneous innervation in impaired diabetic wound healing. Transl Res. 2021;236:87-108. [16] ELEFTHERIADOU I, SAMAKIDOU G, TENTOLOURIS A, et al. Nonpharmacological Management of Diabetic Foot Ulcers: An Update. Int J Low Extrem Wounds. 2021;20(3):188-197. [17] 谷涌泉.中国糖尿病足诊治指南[J].中国临床医生杂志,2020,48(1): 19-27. [18] YANG S, HU L, HAN R, et al. Neuropeptides, Inflammation, Biofilms, and diabetic Foot Ulcers. Exp Clin Endocrinol Diabetes. 2021;10.1055/a-1493-0458. [19] DING Y, CUI L, ZHAO Q, et al. Platelet-Rich Fibrin Accelerates Skin Wound Healing in Diabetic Mice. Ann Plast Surg. 2017;79(3):15-19. [20] BILGEN F, URAL A, BEKERECIOGLU M. Platelet-rich fibrin: An effective chronic wound healing accelerator. J Tissue Viability. 2021;30(4):616-620. [21] 章登辉.人源富血小板纤维蛋白促进大鼠糖尿病创面愈合的实验研究[D].沈阳:中国医科大学,2019. [22] KUMAR RV, SHUBHASHINI N. Platelet rich fibrin: a new paradigm in periodontal regeneration. Cell Tissue Bank. 2013;14(3):453-463. [23] 刘国涛,王军.中药姜黄在创面治疗中研究进展[J].辽宁中医药大学学报,2020,22(9):118-122. [24] PANCHATCHARAM M, MIRIYALA S, GAYATHRI VS, et al. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290(1-2):87-96. [25] 屠卓隆,林才.姜黄素对糖尿病创面的促愈合作用及其机制研究进展[J].中华烧伤杂志,2021,37(4):391-394. [26] YEN YH, PU CM, LIU CW, et al. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9, α-SMA, and collagen. Int Wound J. 2018;15(4):605-617. [27] GENG K, MA X, JIANG Z, et al. Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory. Front Pharmacol. 2021;12:653940. [28] GALINIAK S, BIESIADECKI M, CZUBAT B, et al. Antiglycation activity of curcumin. Advances in Hygiene and Experimental Medicine. 2019;73: 182-188. [29] CABEZA L, ORTIZ R, PRADOS J, et al. Improved antitumor activity and reduced toxicity of doxorubicin encapsulated in poly(ε-caprolactone) nanoparticles in lung and breast cancer treatment: An in vitro and in vivo study. Eur J Pharm Sci. 2017;102:24-34. [30] 王太兵,李颖,贾卓翰,等.白蛋白/透明质酸纳米颗粒制备及递送顺铂效果[J].功能高分子学报.doi: 10.14133/j.cnki.1008-9357. 20210318001 [31] 陶胜岩,王梅,翟光喜.基于透明质酸的刺激响应纳米给药系统的研究进展[J].中国新药与临床杂志,2021,40(5):330-335. [32] WANG H, GU Y, HUANG L, et al. Effectiveness of fire needle combining with moist healing dressing to promote the growth of granulation tissue in chronic wounds: A case report. Int J Nurs Sci. 2020;7(3):386-390. [33] NI Z, SUN J, QI S. Application of Easy Wet Healing Therapy for Chronic Noninfectious Wounds in Limbs. Int J Low Extrem Wounds. 2020; 1534734620924260. [34] 苏金玲.湿性敷料在慢性伤口中的应用研究进展[J].微创医学,2019, 14(3):364-366. [35] QIAN Z, WANG H, BAI Y, et al. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl Mater Interfaces. 2020;12(50):55659-55674. [36] 陈琳,宁宁,陈慧,等.皮肤创伤湿性愈合治疗的临床效果观察[J].华西医学,2015,30(10):1810-1813. [37] 杨群英.糖尿病足的临床特点及P物质在糖尿病足溃疡愈合中的作用[D].广州:南方医科大学,2011. [38] 杨沛瑯.中性粒细胞NETs致糖尿病创面难愈及机制研究[D].上海:上海交通大学,2017. [39] WANG Q, ZHU G, CAO X, et al. Blocking AGE-RAGE Signaling Improved Functional Disorders of Macrophages in Diabetic Wound. J Diabetes Res. 2017;2017:1428537. [49] 刘宸.富血小板血浆对糖尿病大鼠创面巨噬细胞浸润变化的影响[D].广州:南京医科大学,2014. [41] WEAR-MAGGITTI K, LEE J, CONEJERO A, et al. Use of topical sRAGE in diabetic wounds increases neovascularization and granulation tissue formation. Ann Plast Surg. 2004;52(5):519-522. [42] HAN Y, SUN T, TAO R, et al. Clinical application prospect of umbilical cord-derived mesenchymal stem cells on clearance of advanced glycation end products through autophagy on diabetic wound. Eur J Med Res. 2017;22(1):11. [43] LI Q, XIA S, YIN Y, et al. miR-5591-5p regulates the effect of ADSCs in repairing diabetic wound via targeting AGEs/AGER/JNK signaling axis. Cell Death Dis. 2018;9(5):566. [44] OLEKSON MP, FAULKNOR RA, HSIA HC, et al. Soluble Receptor for Advanced Glycation End Products Improves Stromal Cell-Derived Factor-1 Activity in Model Diabetic Environments. Adv Wound Care (New Rochelle). 2016;5(12):527-538. |
| [1] | LIU Danni, SUN Guanghua, ZHOU Guijuan, LIU Hongya, ZHOU Jun, TAN Jinqu, HUANG Xiarong, PENG Ting, FENG Wei-bin, LUO Fu. Effect of electroacupuncture on apoptosis of neurons in cerebral cortex of rats with cerebral ischemia-reperfusion injury at "Shuigou" and "Baihui" points [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [2] | Xiang Xinjian, Liu Fang, Wu Liangliang, Jia Daping, Tao Yue, Zhao Zhengnan, Zhao Yu. High-dose vitamin C promotes the survival of autologous fat transplantation in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1242-1246. |
| [3] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | He Junjun, Huang Zeling, Hong Zhenqiang. Interventional effect of Yanghe Decoction on synovial inflammation in a rabbit model of early knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 694-699. |
| [6] | Liu Yiyi, Qiu Junqiang, Yi Longyan, Zhou Cailiang. Effect of resistance training on interleukin-6 and C-reactive protein in middle-age and elderly people: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 804-812. |
| [7] | Wei Zhoudan, Li Wenjin, Zhu Li, Wang Yu, Zhao Jiaoyang, Chen Yanan, Guo Dong, Hao Min. Platelet-rich fibrin as a material for alveolar ridge preservation significantly reduces the resorption of alveolar bone height and width after tooth extraction: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 643-648. |
| [8] | Zhang Mi, Wu Saixuan, Dong Ming, Liu Tingjiao, Niu Weidong. Novel nano-delivery system: engineered small extracellular vesicles [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4417-4422. |
| [9] | Zheng Haisheng, Lan Yuqing, Zhong Xingwu, Ding Hui. Effect of curcumin nanoparticles on proliferation of human retinal pigment epithelial cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4335-4339. |
| [10] | Pan Ziyin , Lu Yi, Jin Liye, Su Dingwen, Shi Hongcan. Latest research progress and application of bio-printing technology in tracheal tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4393-4400. |
| [11] | Wu Siyi, Wu Qiong. Effect of adipose-derived stem cell transplantation on the immune microenvironment of adipose tissue in aged mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3773-3778. |
| [12] | Qiao Linghui, Yuan Tao, Han Jie, Wang Guancheng, Gu Yanglin. Screening and biological function analysis of inflammation-related circRNAs in synovial tissue of patients with primary knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3683-3690. |
| [13] | Yang Wei, Yuan Puwei, Du Longlong, Li Xuefeng, Gao Qimeng, Han Qingmin. Bioinformatics analysis of gene expression profile of peripheral blood lymphocytes in patients with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3706-3713. |
| [14] | Su Jianqing, Sun Bo, Ding Yunrong, Liu Guangming, Ji Wei, Jiang Enyu, Yang Jiayu. Preparing a rabbit model of synovitis in knee osteoarthritis based on the “injury-repair-reinjury” method [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3738-3743. |
| [15] | Xiakeerzhati•Xiaohalati, Wang Xiaobei, Wang Lin. Nanoparticles: a novel strategy for the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3566-3572. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||