Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (6): 948-956.doi: 10.3969/j.issn.2095-4344.4003
Previous Articles Next Articles
Intravenous, topical tranexamic acid alone or their combination in total knee arthroplasty: a meta-analysis of randomized controlled trials
Huang Dengcheng1, Wang Zhike1, Cao Xuewei2
- 1Second Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; 2Third Department of Orthopedics, Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou 510000, Guangdong Province, China
-
Received:2020-04-03Revised:2020-04-14Accepted:2020-05-09Online:2021-02-28Published:2020-12-05 -
Contact:Cao Xuewei, MD, Chief physician, Third Department of Orthopedics, Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou 510000, Guangdong Province, China -
About author:Huang Dengcheng, Master candidate, Second Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China -
Supported by:the Special Project in Guangdong Provincial Hospital of Traditional Chinese Medicine, No. 2017KT1334
CLC Number:
Cite this article
Huang Dengcheng, Wang Zhike, Cao Xuewei. Intravenous, topical tranexamic acid alone or their combination in total knee arthroplasty: a meta-analysis of randomized controlled trials[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 948-956.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
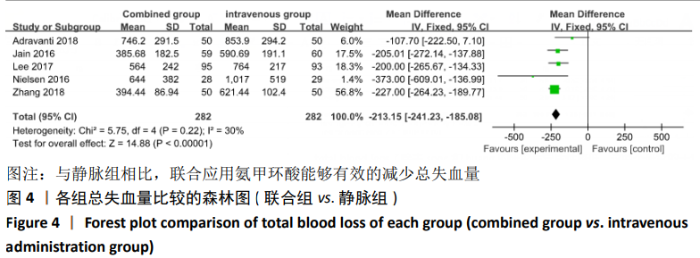
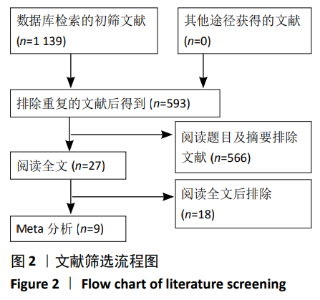
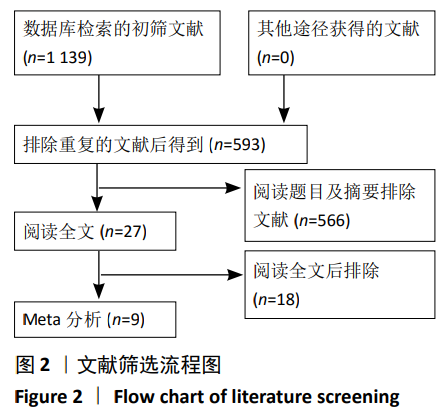
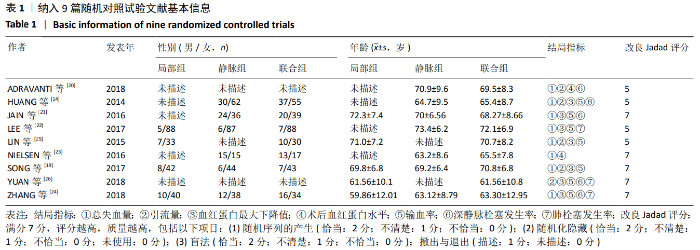
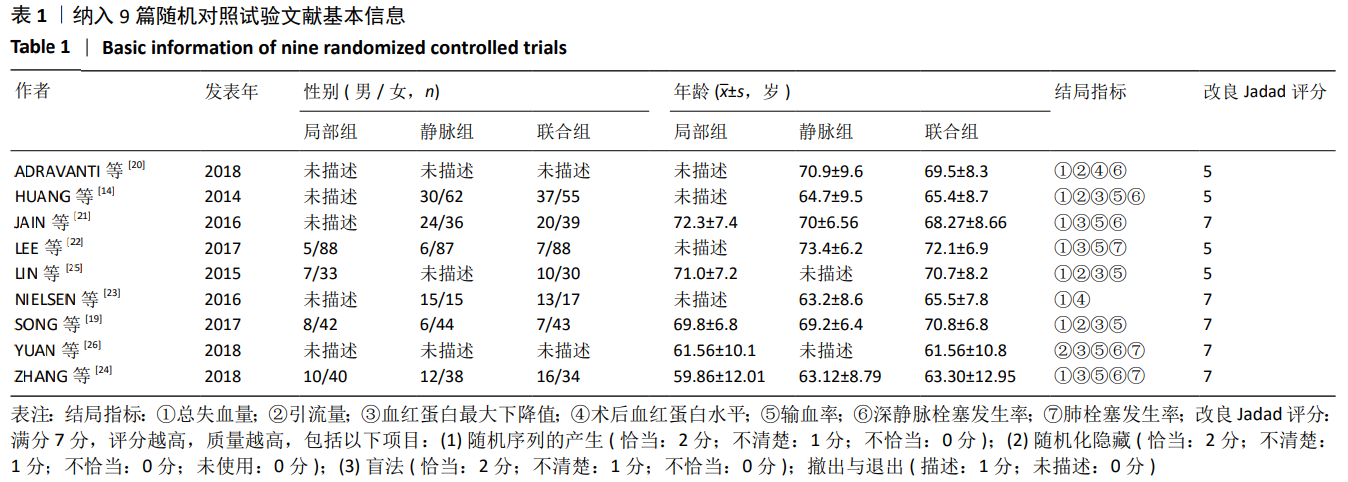
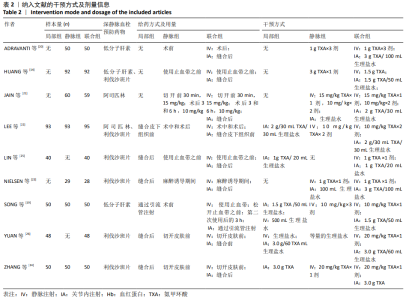
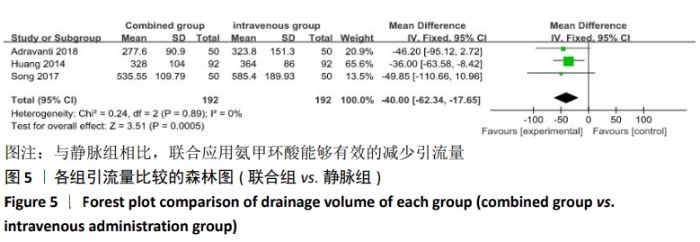
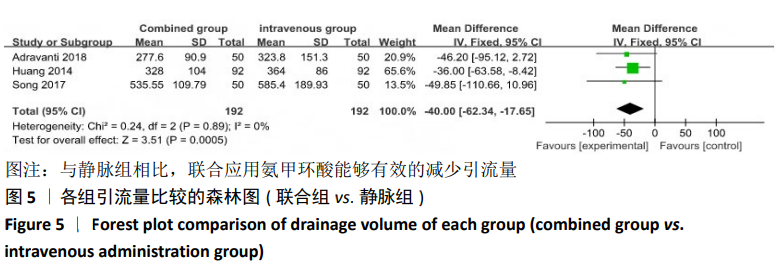
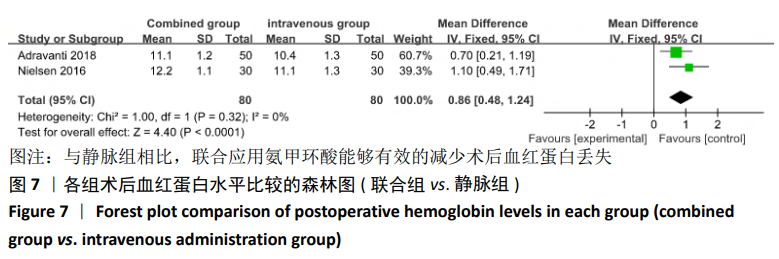
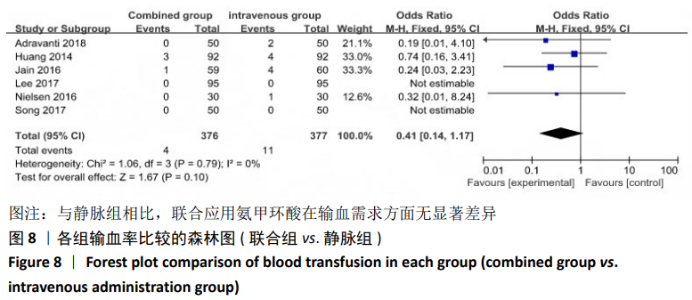
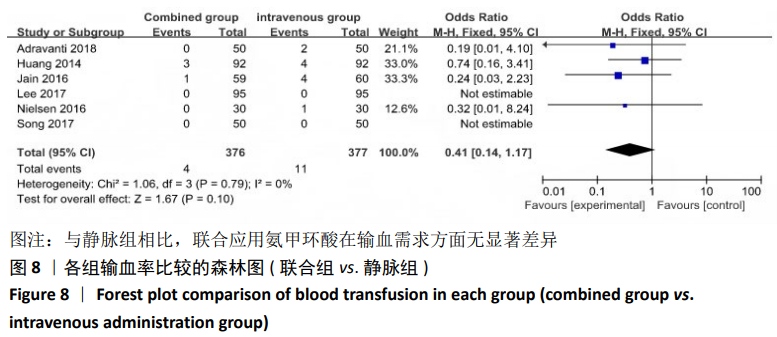
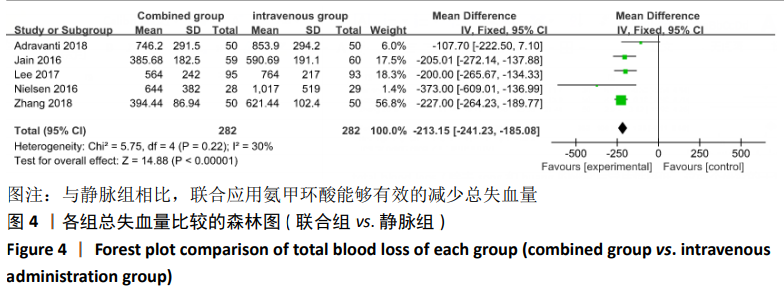
2.4 Meta分析结果 2.4.1 联合用药与静脉给药对比 (1) 手术时间:纳入的研究中有5篇文献报道了2种干预方式的手术时间[14,19,21,23-24],Meta分析结果显示,研究效应量存在轻度异质性(I2=39%,P=0.16),因此采用固定效应模型。两组相比,差异有显著性意义(MD=1.25,95%CI: -0.11-2.62,P=0.07),不同给药方式的手术时间无显著差异。 (2) 总失血量:因SONG等[19]和HUANG等[14]的静脉组给药剂量与联合组总量相同,剪切法分析发现在删除这2项研究后有着显著变化,因此去除这2项试验;纳入的研究中有5篇文献报道了2种干预方式的总失血量[20-24],Meta分析结果显示,研究效应量存在轻度异质性(I2=30%,P=0.22),因此采用固定效应模型。两组相比差异有显著性意义(MD=-213.15,95%CI:-241.23至-185.08,P < 0.000 01),见图4,因此可认为联合用药可显著降低全膝关节置换的总失血量。"
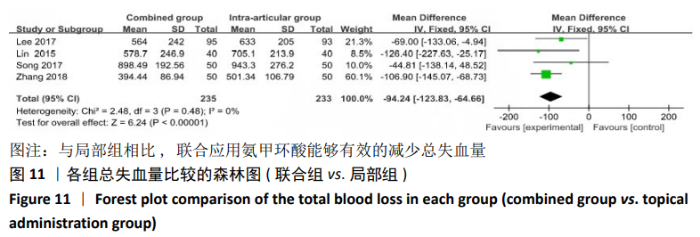
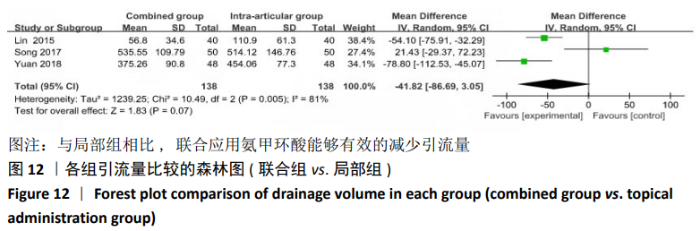
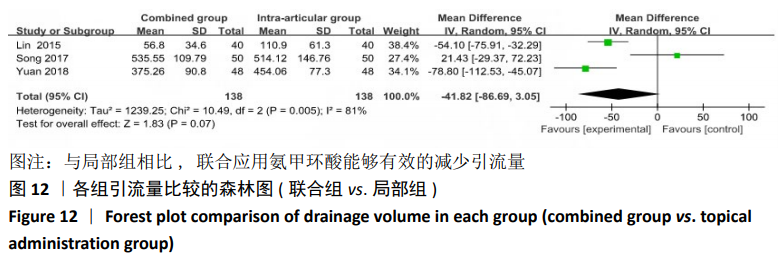
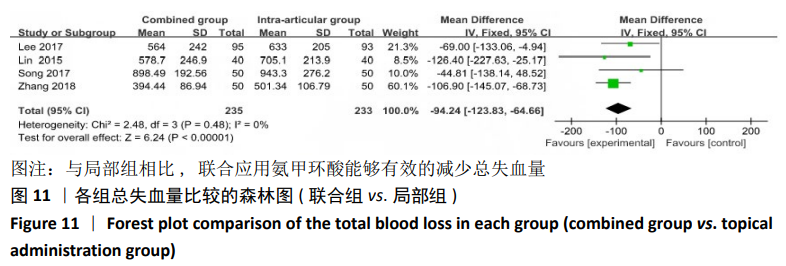
2.4.2 联合用药与局部给药对比 (1)手术时间:纳入的研究中有3篇文献报道了2种干预方式的手术时间[19,24-25],Meta分析结果显示,研究效应量存在轻度异质性(I2=22%,P=0.28),因此采用固定效应模型。两组相比差异有显著性意义(MD=-0.87,95%CI: -3.78-2.04,P=0.56),不同给药方式的手术时间无显著差异。 (2)总失血量:纳入的研究中有4篇文献报道了2种干预方式的总失血量[19,22,24-25],Meta分析结果显示,研究效应量存在无异质性(I2=0%,P=0.48),因此采用固定效应模型。两组相比差异有显著性意义(MD= -94.24,95%CI:-123.83至-64.66,P < 0.000 01),见图11。因此可认为,与仅局部给药方式相比,联合用药可显著降低全膝关节置换的总失血量。"
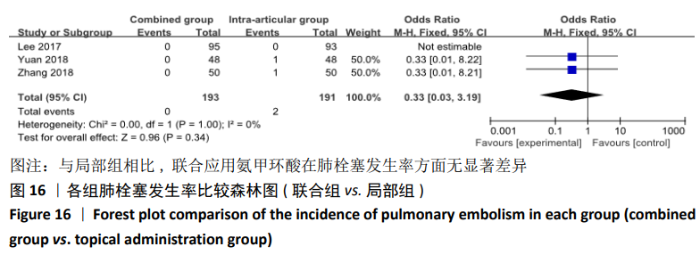
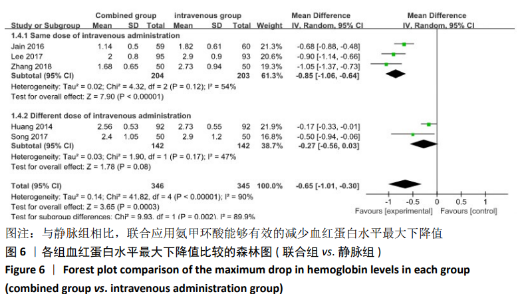
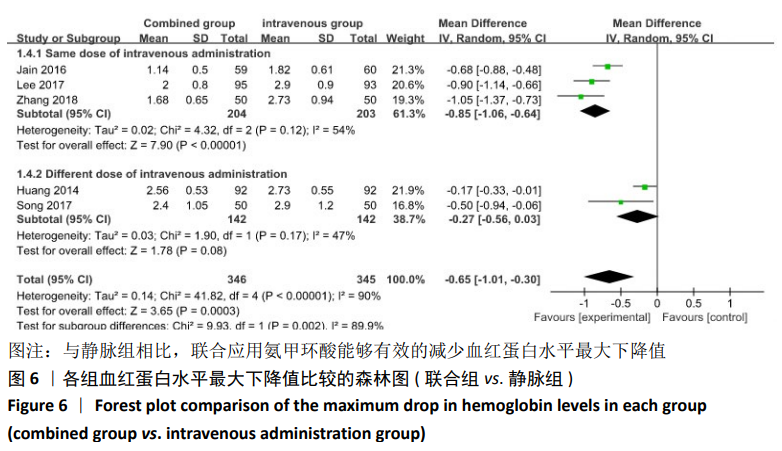
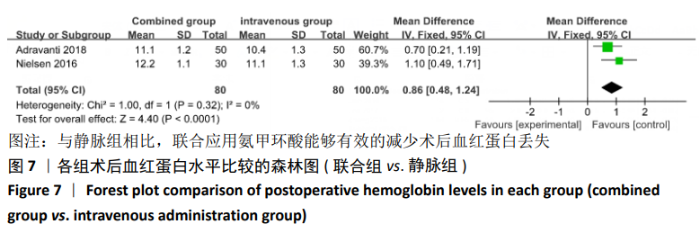
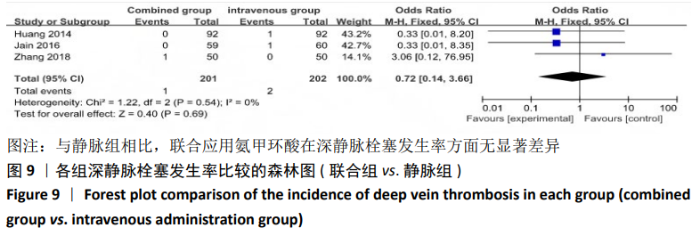
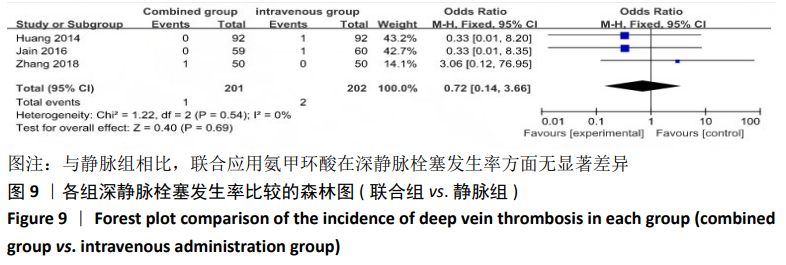
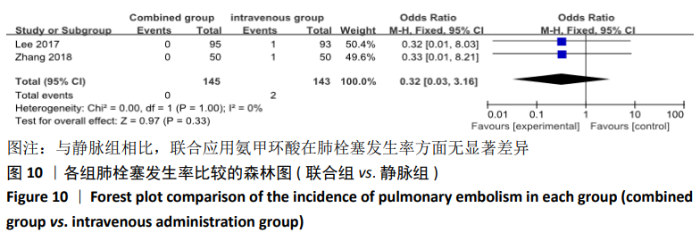
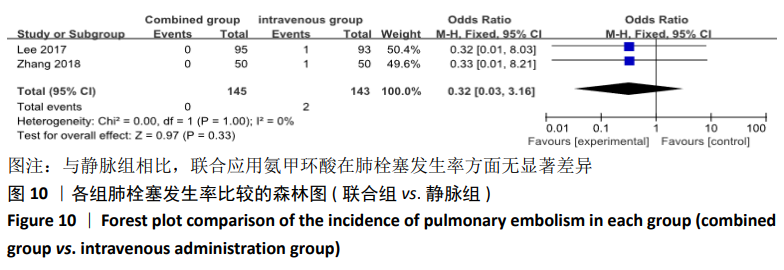
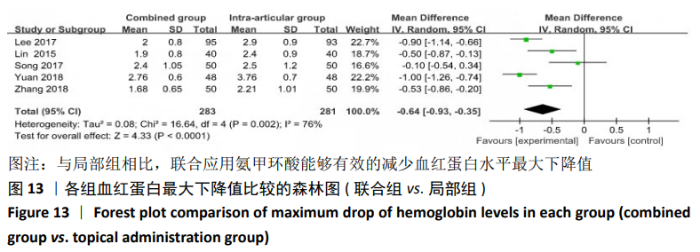
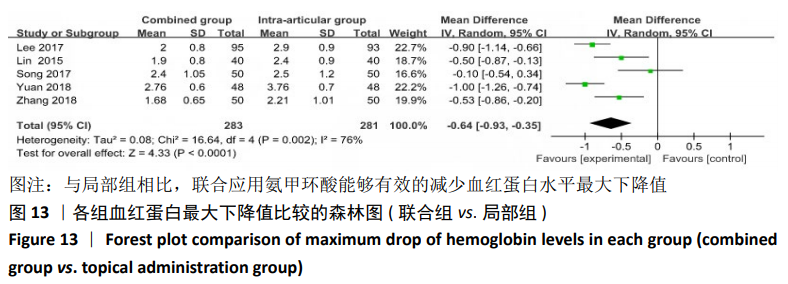
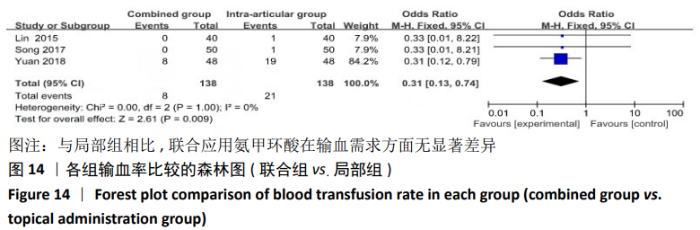
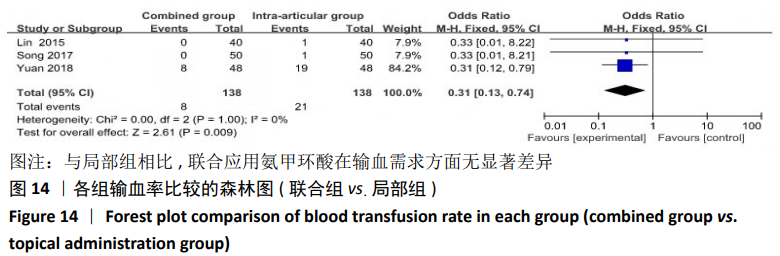
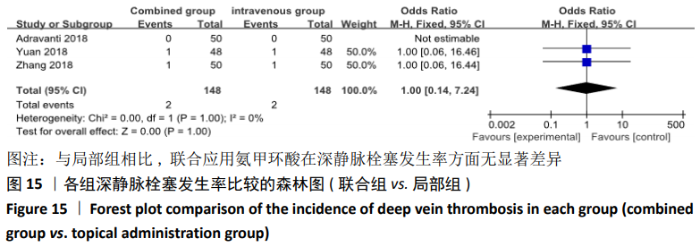
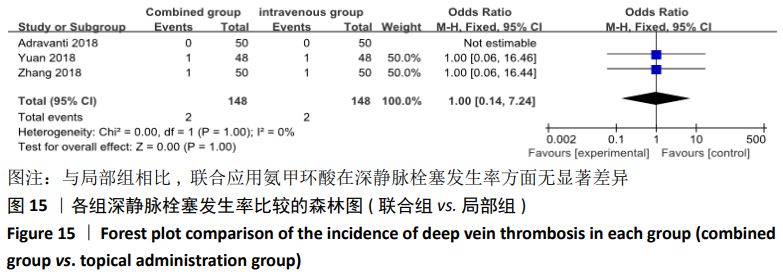
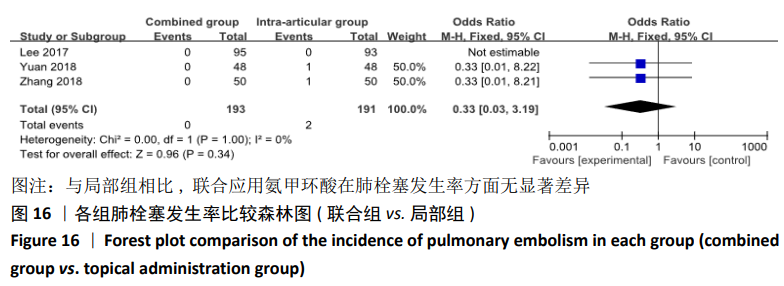
(7)肺栓塞发生率:有3篇文献报道了2种干预方式的肺栓塞发生 率[22, 24,26],汇总显示联合组与局部氨甲环酸组之间的研究效应量无异质性(I2=0%,P=1.00),因此采用固定效应模型。两组间静脉栓塞的发生率差异无显著性意义(OR=0.33,95%CI:0.03-3.19,P=0.34),见图16,局部组的发生率为1.04%,可认为氨甲环酸的应用没有引起静脉栓塞事件发生率的升高。 2.4.3 敏感性分析 此次Meta分析结果显示,在联合组与静脉组应用氨甲环酸的对比中,总失血量和血红蛋白水平最大下降值的结果异质性较大。因此文章利用剪切法对纳入研究的异质性进行分析,逐一筛选后发现异质性来源于SONG等[19]和HUANG等[14]的研究,考虑异质性来源是这2项研究的静脉组给药剂量与联合组用药总剂量相同,因此不予纳入这2项试验;而逐一剔除研究后术后血红蛋白水平最大下降值异质性仍较高,进行亚组分析可减小亚组异质性,但总异质性仍较高,组间差异有显著性意义。在联合组与局部组应用氨甲环酸的对比中,引流量和血红蛋白最大下降值的结果异质性较大,对纳入的研究进行逐一剔出,未见异质性降低,详细阅读纳入的各项研究,组间差异有显著性意义。说明此Meta分析的结果较为可靠,异质性来源可能与各研究中心的手术技巧相关,同时,局部组中的一项研究为全膝关节置换翻修,可能与此也有一定的关系。 2.5 发表偏倚分析 根据Cochrane手册的建议,漏斗图进行发表偏倚评价需该指标Meta分析纳入的研究数不应少于10篇文献。否则会因为该指标纳入的研究数过少,从而降低漏斗图的检验能力,否则无法判断不对称的真实性。此文献的各项研观察指标均未达到10篇文献,所以未进行发表偏倚漏斗图分析。 "
| [1] KURTZ S, ONG K, LAU E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007; 89(4):780-785. [2] KIM DH, LEE GC, LEE SH, et al. Comparison of blood loss between neutral drainage with tranexamic acid and negative pressure drainage without tranexamic acid following primary total knee arthroplasty. Knee Surg Relat Res. 2016;28(3):194-200. [3] SEOL YJ, SEON JK, LEE SH, et al. Effect of tranexamic acid on blood loss and blood transfusion reduction after total knee arthroplasty. Knee Surg Relat Res. 2016; 28(3):188-193. [4] LEIGHEB M, POGLIACOMI F, BOSETTI M, et al. Postoperative blood salvage versus allogeneic blood transfusion in total knee and hip arthroplasty: a literature review. Acta Biomed. 2016;87(1):6-14. [5] SCHREIBER GB, BUSCH MP, KLEINMAN SH, et al. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334(26):1685-1690. [6] LIU D, DAN M, MARTINEZ MS, et al. Blood Management Strategies in Total Knee Arthroplasty. Knee Surg Relat Res. 2016; 28(3):179-187. [7] SAMUJH C, FALLS TD, WESSEL R, et al. Decreased blood transfusion following revision total knee arthroplasty using tranexamic acid. J Arthroplasty. 2014;29(9):182-185. [8] SHIMIZU M, KUBOTA R, NASU M, et al. The influence of tourniquet during total knee arthroplasty on perioperative blood loss and postoperative complications. Jpn J Anesthesiol. 2016;65(2):131-135. [9] FU Y, SHI ZG, HAN B, et al. Comparing efficacy and safety of 2 methods of tranexamic acid administration in reducing blood loss following total knee arthroplasty: a meta-analysis. Medicine. 2016;95(50):e5583. [10] WANG SQ, GAO XX, AN Y. Topical versus intravenous tranexamic acid in total knee arthroplasty: a meta-analysis of randomized controlled trials. Int Orthop. 2017;41(4):739-748. [11] CHAROENCHOLVANICH K, SIRIWATTANASAKUL P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res. 2011;469(10):2874-2880. [12] TAN J, CHEN H, LIU Q, et al. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887. [13] WONG J, ABRISHAMI A, EL BEHEIRY H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010; 92(15):2503-2513. [14] HUANG Z, MA J, SHEN B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(12): 2342-2346. [15] MACHIN JT, BATTA V, SOLER JA, et al. Comparison of intra-operative regimes of tranexamic acid administration in primary total hip replacement. Acta Orthop Belg. 2014;80(2):228-233. [16] WU YG, ZENG Y, YANG TM, et al. The efficacy and safety of combination of intravenous and topical tranexamic acid in revision hip arthroplasty: a randomized, controlled trial. J Arthroplasty. 2016;31(11):2548-2553. [17] XIE J, MA J, YUE C, et al. Combined use of intravenous and topical tranexamic acid following cementless total hip arthroplasty: a randomised clinical trial. Hip int. 2016; 26(1):36-42. [18] YI Z, BIN S, JING Y, ZONGKE Z, et al. Tranexamic acid administration in primary total hip arthroplasty: a randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am. 2016;98(12):983-991. [19] SONG EK, SEON JK, PRAKASH J, et al. Combined administration of IV and topical tranexamic acid is not superior to either individually in primary navigated TKA. J Arthroplasty. 2017;32(1):37-42. [20] ADRAVANTI P, DI SE, CALAFIORE G, et al. A prospective, randomized, comparative study of intravenous alone and combined intravenous and intraarticular administration of tranexamic acid in primary total knee replacement. Arthroplasty Today. 2018;4(1):85-88. [21] JAIN NP, NISTHANE PP, SHAH NA. Combined administration of systemic and topical tranexamic acid for total knee arthroplasty: can it be a better regimen and yet safe? A Randomized Controlled Trial. J Arthroplasty. 2016;31(2):542-547. [22] LEE SY, CHONG S, BALASUBRAMANIAN D, et al. What is the Ideal Route of Administration of Tranexamic Acid in TKA? A Randomized Controlled Trial. Clin Orthop Relat Res. 2017;475(8):1987-1996. [23] NIELSEN CS, JANS Ø, ØRSNES T, et al. Combined intra-articular and intravenous tranexamic acid reduces blood loss in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2016;98(10):835-841. [24] ZHANG YM, YANG B, SUN X, et al. Combined intravenous and intra-articular tranexamic acid administration in total knee arthroplasty for preventing blood loss and hyperfibrinolysis: a randomized controlled trial. Medicine. 2019;98(7):e14458. [25] LIN SY, CHEN CH, FU YC, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30(5):776-780. [26] YUAN X, WANG J, WANG Q, et al. Synergistic effects of intravenous and intra-articular tranexamic acid on reducing hemoglobin loss in revision total knee arthroplasty: a prospective, randomized, controlled study. Transfusion. 2018;58(4):982-988. [27] ROSSAINT R, BOUILLON B, CERNY V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. [28] KOZEK-LANGENECKER SA, AFSHARI A, ALBALADEJO P, et al. Management of severe perioperative bleeding: guidelines from the european society of anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270-382. [29] FERRARIS VA, BROWN JR, DESPOTIS GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thoracic Surg. 2011;91(3):944-982. [30] YIN D, DELISLE J, BANICA A, et al. Tourniquet and closed-suction drains in total knee arthroplasty. No beneficial effects on bleeding management and knee function at a higher cost. Orthop Traumatol Surg Res. 2017;103(4):583-589. [31] LUNEBOURG A, PARRATTE S, OLLIVIER M, et al. Are revisions of unicompartmental knee arthroplasties more like a primary or revision TKA? J Arthroplasty. 2015;30(11): 1985-1989. [32] CHANG CW, LAN SM, TAI TW, et al. An effective method to reduce ischemia time during total knee arthroplasty. J Formos Med Assoc. 2012;111(1):19-23. [33] ZHANG W, LIU A, HU D, et al. Effects of the timing of tourniquet release in cemented total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2014;9:125. [34] JIANG FZ, ZHONG HM, HONG YC, et al. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(1):110-123. [35] DUNN CJ, GOA KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005-1032. [36] YU X, LI W, XU P, et al. Safety and efficacy of tranexamic acid in total knee arthroplasty. Med Sci Monit. 2015;21:3095-3103. [37] EVANGELISTA PJ, AVERSANO MW, KOLI E, et al. Effect of tranexamic acid on transfusion rates following total joint arthroplasty: a cost and comparative effectiveness analysis. Orthop Clin North Am. 2017;48(2): 109-115. [38] ZHANG LK, MA JX, KUANG MJ, et al. The efficacy of tranexamic acid using oral administration in total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res. 2017;12(1):159. [39] GUZEL Y, GURCAN OT, GOLGE UH, et al. Topical tranexamic acid versus autotransfusion after total knee arthroplasty. J Orthopaedic Surg. 2016; 24(2):179-182. [40] GOYAL N, CHEN DB, HARRIS IA, et al. Clinical and financial benefits of intra-articular tranexamic acid in total knee arthroplasty. J Orthopaedic Surg. 2016;24(1):3-6. [41] LI GL, LI YM. Oral tranexamic acid can reduce blood loss after total knee and hip arthroplasty: a meta-analysis. Int J Surg. 2017;46:27-36. [42] YANG ZG, CHEN WP, WU LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. [43] PITTA M, ZAWADSKY M, VERSTRAETE R, et al. Intravenous administration of tranexamic acid effectively reduces blood loss in primary total knee arthroplasty in a 610-patient consecutive case series. Transfusion. 2016;56(2):466-471. [44] XIE J, MA J, KANG P, et al. Does tranexamic acid alter the risk of thromboembolism following primary total knee arthroplasty with sequential earlier anticoagulation? A large, single center, prospective cohort study of consecutive cases. Thromb Res. 2015;136(2):234-238. [45] WEI W, DANG S, DUAN D, et al. Comparison of intravenous and topical tranexamic acid in total knee arthroplasty. BMC Musculoskelet Disord. 2018;19(1):191. [46] PRAKASH J, SEON JK, PARK YJ, et al. A randomized control trial to evaluate the effectiveness of intravenous, intra-articular and topical wash regimes of tranexamic acid in primary total knee arthroplasty. J Orthop Surgery (Hong Kong). 2017;25(1): 2309499017693529. [47] MAY JH, RIESER GR, WILLIAMS CG, et al. The assessment of blood loss during total knee arthroplasty when comparing intravenous vs intracapsular administration of tranexamic acid. J Arthroplasty. 2016;31(11): 2452-2457. [48] SPANYER J, PATEL J, EMBERTON E, et al. Topical tranexamic acid in total knee arthroplasty patients with increased thromboembolic risk. J Knee Surg. 2017; 30(5):474-478. [49] YUAN ZF, YIN H, MA W, et al. The combined effect of administration of intravenous and topical tranexamic acid on blood loss and transfusion rate in total knee arthroplasty: Combined tranexamic acid for TKA. Bone Joint Res. 2016;5(8):353-361. [50] SUN Q, LI J, CHEN J, et al. Comparison of intravenous, topical or combined routes of tranexamic acid administration in patients undergoing total knee and hip arthroplasty: a meta-analysis of randomised controlled trials. BMJ Open. 2019;9(1):e024350. [51] XIONG H, LIU Y, ZENG Y, et al. The efficacy and safety of combined administration of intravenous and topical tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. BMC Musculoskeletal Disorders. 2018;19 (1):321. [52] WANG Z, SHEN X. The efficacy of combined intra-articular and intravenous tranexamic acid for blood loss in primary total knee arthroplasty: a meta-analysis. Medicine. 2017;96(42):e8123. |
| [1] | Li Dadi, Zhu Liang, Zheng Li, Zhao Fengchao. Correlation of total knee arthroplasty efficacy with satisfaction and personality characteristics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1346-1350. |
| [2] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [3] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [4] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [5] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [6] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [7] | Yuan Jun, Yang Jiafu. Hemostatic effect of topical tranexamic acid infiltration in cementless total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 873-877. |
| [8] | Li Yan, Wang Pei, Deng Donghuan, Yan Wei, Li Lei, Jiang Hongjiang. Electroacupuncture for pain control after total knee arthroplasty: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 957-963. |
| [9] | He Xiangzhong, Chen Haiyun, Liu Jun, Lü Yang, Pan Jianke, Yang Wenbin, He Jingwen, Huang Junhan. Platelet-rich plasma combined with microfracture versus microfracture in the treatment of knee cartilage lesions: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 964-969. |
| [10] | Zhan Fangbiao, Cheng Jun, Zou Xinsen, Long Jie, Xie Lizhong, Deng Qianrong. Intraoperative intravenous application of tranexamic acid reduces perioperative bleeding in multilevel posterior spinal surgery: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 977-984. |
| [11] | Zhong Hehe, Sun Pengpeng, Sang Peng, Wu Shuhong, Liu Yi. Evaluation of knee stability after simulated reconstruction of the core ligament of the posterolateral complex [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 821-825. |
| [12] | Zhao Zhongyi, Li Yongzhen, Chen Feng, Ji Aiyu. Comparison of total knee arthroplasty and unicompartmental knee arthroplasty in treatment of traumatic osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 854-859. |
| [13] | Liu Shaohua, Zhou Guanming, Chen Xicong, Xiao Keming, Cai Jian, Liu Xiaofang. Influence of anterior cruciate ligament defect on the mid-term outcome of fixed-bearing unicompartmental knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 860-865. |
| [14] | Zhang Nianjun, Chen Ru. Analgesic effect of cocktail therapy combined with femoral nerve block on total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 866-872. |
| [15] | Xie Chongxin, Zhang Lei. Comparison of knee degeneration after anterior cruciate ligament reconstruction with or without remnant preservation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 735-740. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||