Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (7): 1120-1125.doi: 10.3969/j.issn.2095-4344.0127
Previous Articles Next Articles
Role of macrophage polarization in periprosthetic osteolysis: its research status and advance
Gong Dong1, 2, Zhen Ping1, Wang Rong2
- 1Department of Orthopedics, Lanzhou General Hospital of Lanzhou Military Region of Chinese PLA , Lanzhou 730000, Gansu Province, China; 2Gansu University of Chinese Medicine, Lanzhou 730000, Gansu Province, China
-
Online:2018-03-08Published:2018-03-08 -
About author:Gong Dong, Studying for master’s degree, Department of Orthopedics, Lanzhou General Hospital of Lanzhou Military Region of Chinese PLA , Lanzhou 730000, Gansu Province, China; Gansu University of Chinese Medicine, Lanzhou 730000, Gansu Province, China -
Supported by:the National Natural Science Foundation of China, No. 81371983
CLC Number:
Cite this article
Gong Dong, Zhen Ping, Wang Rong. Role of macrophage polarization in periprosthetic osteolysis: its research status and advance[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(7): 1120-1125.
share this article
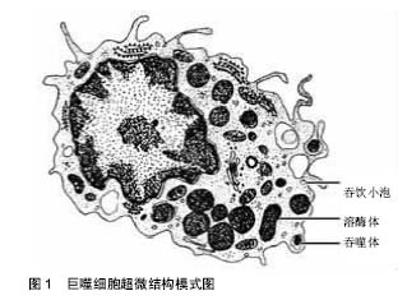
2.1 巨噬细胞极化的相关概念 巨噬细胞是免疫细胞的一种,源自单核细胞,普遍存在于血液、淋巴和组织中,是免疫系统的重要组分(图1)。在病原微生物与衰老细胞的清除、促进和抑制炎症反应、诱发适应性免疫反应及损伤组织的修复与重构过程中,巨噬细胞都起着重要作用[4]。其作为免疫系统中可塑性最强的细胞,经不同病原相关分子模式和损伤相关分子模式的刺激,分裂为具有不同功能的表型(即极化)[5]。根据刺激因子的不同和功能的差异可将巨噬细胞分为经典活化的M1型巨噬细胞(classically activated macrophage)和选择性活化的M2型巨噬细胞(alternatively activated macrophage),两者在不同的疾病病理过程中相互转化,涉及多种机制和因素的共同影响,即参与病原体清除、促进炎症发生,又可修复受损组织、抑制炎症反应。例如,病原体入侵机体时,巨噬细胞被改变的机体微环境准确地极化成M1型,产生大量炎性因子,杀死入侵的生物体并激活适应性免疫。同时这些炎性因子又可诱导巨噬细胞凋亡或向M2型巨噬细胞极化,缓解炎症作用及避免机体过度损伤,进而促进伤口愈合。"
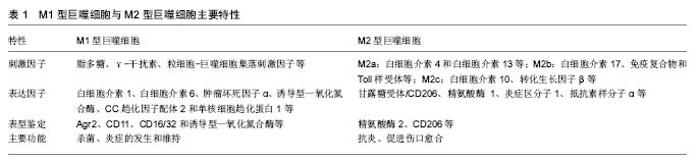
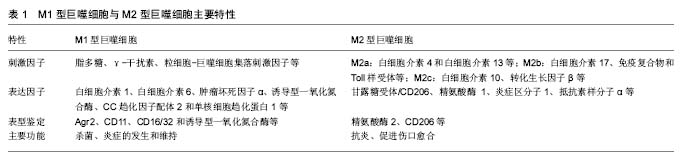
2.1.1 M1、M2功能及其特点 巨噬细胞的M1型和M2型是生理或表型变化的极端状态,这种状态很大程度上受到环境的影响[6]。目前研究较为明确的是,休眠状态的巨噬细胞(M0)可由脂多糖和或γ-干扰素及粒细胞-巨噬细胞集落刺激因子等诱导活化为M1型巨噬细胞,分泌大量白细胞介素1、白细胞介素 6、肿瘤坏死因子α、诱导型一氧化氮合酶等促炎性细胞因子,同时能产生趋化因子,如CC趋化因子配体2、单核细胞趋化蛋白1等吸引Th1细胞[7],促进强烈的Th1免疫反应,参与杀菌、炎症的发生和维持作用[8]。 M2型巨噬细胞主要起抗炎、促进伤口愈合和纤维化、组织修复、血管再生以及促肿瘤生长和浸润等作 用[9],可分为a、b和c 3个亚型,其中M2a可通过白细胞介素4和白细胞介素13激活;M2b型由白细胞介素17、免疫复合物和Toll样受体激活;M2c型可被白细胞介素10、转化生长因子β或糖皮质激素活化[10-13]。免疫调节和吞噬凋亡细胞的反应主要由M2c介导,M2c活化后产生高水平的白细胞介素10、转化生长因子β及低水平白细胞介素12等,抑制T细胞的增殖和活化而下调免疫应答,可通过较强的吞噬清除能力和合成营养因子来减少促炎因子的分泌[5],并高表达甘露糖受体/CD206、精氨酸酶1、炎症区分子1、抵抗素样分子α、类几丁质酶3样分子3等抑炎性细胞因子[14],在粒细胞介导的先天免疫反应及Th2型免疫应答中发挥重要作用。M1、M2主要特性见表1。 2.1.2 M1、M2亚型的鉴定 巨噬细胞亚型的鉴定一般根据其标志物,但因其标志物表达的多样性,如何选择尚无统一标准。Khallou-Laschet等[15]在骨髓源性巨噬细胞向M1、M2型极化的过程中发现,精氨酸酶2在M1中、精氨酸酶1在M2中的表达都明显上调,因此他们认为可以将精氨酸酶作为两型巨噬细胞的替代标志物。有学者用CD206作为标志物检测到M2型巨噬细胞在人类皮下脂肪组织中大量存在[16]。另外,Lumeng等[17]通过流式细胞仪证明CD11可作为M1型巨噬细胞的标志物。而在对M1和M2表型指标进行相对量化的比较时发现,白细胞介素12的分泌、诱导型一氧化氮合酶表达和活性以及膜蛋白CD16/32的表达可用于鉴定M1型巨噬细胞; Arg-1表达和活性、CD206以及DECTIN-1的表达,可用于鉴定M2型巨噬细胞[18]。总之,关于巨噬细胞亚型的鉴定涉及膜蛋白、细胞因子、代谢酶等多个方面,并在不同的疾病范围使用不同的标志物,虽未完全明确,但一般以诱导型一氧化氮合酶和Arg-1的应用较为广泛,其他标志物尚待进一步研究。 2.1.3 巨噬细胞极化的信号通路 巨噬细胞的极化是一个多因子相互作用的复杂过程,受到多种信号分子及其通路的调控,相关过程可能成为疾病治疗的标靶,因此巨噬细胞极化信号通路的研究对巨噬细胞参与的相关疾病的治疗具有重要意义。 JAK/STAT信号通路:JAK/STAT信号通路与多种细胞因子诱导的生物学应答有关,受内外环境刺激因素的调节。研究显示,JAK/STAT信号通路的激活显著影响巨噬细胞极化[19]。其中γ-干扰素是最重要的通过JAK/STAT激活巨噬细胞的内源性因子,主要激活JAK并使STAT1磷酸化,诱导巨噬细胞向M1极化。而白细胞介素4主要通过JAK/STAT6途径,使磷酸化的STAT6形成二聚体,其与受体的亲和力降低,分离后转位至核内直接与相关转录因子 Kruppel样因子4(KLF4)、过氧 化物酶增殖物激活受体γ结合,启动基因转录调控M2极化[20]。 PI3K/AKT信号通路:P13K/AKT信号通路在参与巨噬细胞的激活和基因表达中扮演重要角色[21]。细菌、脂多糖等引起的感染和内毒素型休克均可激活PI3K,分别调节AKT1与AKT2的活化。Xu等[22]研究发现AKT1敲除后的小鼠巨噬细胞偏向于M1的极化,而AKT2敲除后偏向于M2型极化。核因子κB是PI3K/AKT信号通路中的关键信号因子,其激活可导致M1极化,引起小鼠骨质破坏[23-24]。另外,Gadang等[25]发现骨形态生成蛋白7可激活PI3K,导致下游的AKT1和mTOR激活,促进巨噬细胞向M2型极化。 JNK信号通路:JNK信号通路属于MAPKs超家族中的一员,参与调节细胞的许多代谢过程。MAP3K、核因子κB以及CCR2都可活化JNK信号通路,进而导致AP-1的上调以及M1型巨噬细胞相关产物的表达[25-27]。而Kang等[28]发现,脂肪细胞分泌Th2细胞因子,如白细胞介素13,可上调巨噬细胞过氧化物酶受体,抑制JNK信号通路,而使得巨噬细胞向M2极化。 2.2 巨噬细胞极化与骨溶解 人工关节置换术后随着假体置入时间的延长会在体内产生大量的磨损颗粒,这些理化性质稳定的磨损颗粒在与其周围组织细胞接触或被巨噬细胞吞噬后可诱导假体周围慢性炎症反应,而巨噬细胞是松动的假体周围最初聚集于有炎性反应位置 的细胞之一[29],聚集的巨噬细胞进一步分泌趋化因子和促炎因子,抑制成骨细胞并激活破骨细胞,最终导致假体周围骨溶解以及无菌性松动的发生。虽然巨噬细胞在骨科植入物相关的慢性炎症中起到十分关键的作用已经被有所认识[30],但是从巨噬细胞极化的角度所做的探索还相对较少,以下从假体周围巨噬细胞的极化状态、磨损颗粒与巨噬细胞极化以及巨噬细胞极化与骨溶解的治疗方面来做一叙述。 2.2.1 假体周围巨噬细胞的极化状态 目前并无研究能够直接表征假体周围巨噬细胞极化状态,多是根据M1和M2型巨噬细胞表达的相关标记物来进行假设、推测及验证。有学说认为,由于单核细胞/巨噬细胞前体的可塑性以及巨噬细胞活化的微环境,在假体周围巨噬细胞可能最终极化为M1或M2某一特定的表型[31-32]。也有研究认为,骨溶解中的巨噬细胞极化状态可能与巨噬细胞极化的表观遗传调控相关[33]。具体而言,磨损颗粒诱导巨噬细胞黏附并向M1型极化,促进急性炎症的发生,这种状态可能克服了由交替激活的M2型巨噬细胞所起的抗炎作用,最终表现为M1型极化状态[34]。与骨关节炎组织作为对照组时,Koulouvaris等[35]用实时定量RT-PCR法在松动的假体周围发现M2巨噬细胞相关标记物几丁质酶1和CC趋化因子配体18的表达增加,而肿瘤坏死因子α和白细胞介素6等M1相关标记物则无差异,所以作者认为至少在骨溶解的结束阶段以M2巨噬细胞的活化为主。当用人类白细胞抗原DR及CD163分别作为M1和M2的标记时,Rao等[36]发现假体周围有M1/M2的比例增高,并且相比于初次关节置换术,在需要翻修的关节组织中这种情况更为明显。在磨损颗粒与Toll样受体介导炎症的相关研究中发现,超高分子量聚乙烯可与组织细胞的TLR2和TLR2/1聚体结合激活相关炎症信号通路[37],钴离子也已被证明能够直接结合并激活TLR4信号[38-40],这些证据进一步支持了M1型巨噬细胞在假体周围炎症中作用。另外也有观点认为,可能存在其他不同于经典的巨噬细胞极化和激活信号途经来解释假体周围组织巨噬细胞表型。因此,有关假体周围巨噬细胞极化表型目前尚存争议,但考虑到巨噬细胞激活、生物材料颗粒和假体周围组织的炎症性质,多数研究倾向于M1型巨噬细胞处于假体周围巨噬细胞极化的主导地位。 2.2.2 磨损颗粒、微环境与巨噬细胞极化 磨损颗粒的产生主要与关节假体材料的选择有关,常见的磨损颗粒有聚乙烯颗粒(高交联聚乙烯颗粒、超高分子聚乙烯颗粒)、金属颗粒(钴铬合金、钛合金)、陶瓷等,这些磨损颗粒均可引起相似的细胞免疫应答反应引起巨噬细胞极化。在Joseph等[41]的体外实验中发现用PMMA的磨损颗粒处理的巨噬细胞有M1相关产物肿瘤坏死因子α和白细胞介素1β表达增加。来源于超高分子量聚乙烯的烷烃聚合物可直接通过Toll样受体通路激活核因子κB使巨噬细胞趋向M1极化[42]。钴、钛等金属离子也已被证明诱导炎症并影响巨噬细胞极化状态[38,43],因此有学者引入“离子病”(Ion disease)的概念,补充“粒子病”( particle disease)在假体松动中作用的理解[44]。关节置换及翻修术后,在假体或骨水泥与骨之间形成一层界膜,其内大量的磨损颗粒、巨噬细胞、成纤维细胞、淋巴细胞和这些细胞所分泌的促炎及抗炎细胞因子共同构成了假体周围的微环境。巨噬细胞可根据假体周围局部微环境所指示的信号,动态转变为不同的表型,这种巨噬细胞可塑性最好的例子就是巨噬细胞极化现象[45]。除了磨损颗粒的特性,巨噬细胞的所处的极化状态以及微环境决定的巨噬细胞表型是确定巨噬细胞对磨损颗粒刺激反应方式的另外因素[29,41]。研究无菌性松动的假体周围的巨噬细胞极化因子特征时,Jämsen 等[46]发现白细胞介素10、巨噬细胞集落刺激因子、白细胞介素8、CC趋化因子配体2-4、CXCL9-10、CC趋化因子配体22、抗酒石酸酸性磷酸酶、cathepsin K等细胞因子有明显的增高相比较于骨关节炎,而经典的巨噬细胞极化细胞因子γ-干扰素、粒细胞-巨噬细胞集落刺激因子、白细胞介素4、白细胞介素13以及白细胞介素17A并没有优先表达,作者认为翻修假体周围的巨噬细胞表型有M1和M2的共同特点,且表型可能受局部磨损颗粒和全身因素的共同调节,而不只是典型的极化因子。在最新的相关研究中进一步确认了促炎递质、趋化因子和TRL4与M1极化之间的正相关关系,并认为γ-干扰素和TRL4在骨溶解过程中起到重要作用[47]。可见,磨损颗粒、假体周围微环境与巨噬细胞极化密切相关,并可能受到全身多种因素的影响。 2.2.3 巨噬细胞极化与骨溶解的治疗 越来越多的研究表明,在磨损颗粒诱导的假体周围骨溶解及无菌性松动过程中,不仅磨损颗粒本身,且巨噬细胞极化也起着至关重要的作用。根据不同表型巨噬细胞所具有的不同功能,调节巨噬细胞极化(即诱导巨噬细胞向具有的抗炎、组织修复、血管再生等作用M2型极化,抑制其向具有促炎作用的M1型极化)是减轻磨损颗粒引起的炎症和骨溶解的一个可行策略。此前就有报道指出白细胞介素4能够减少聚甲基丙烯酸甲酯颗粒刺激巨噬细胞产生的肿瘤坏死因子α、白细胞介素1β和粒细胞-巨噬细胞集落刺激因子,且和白细胞介素4的剂量相关[29、48],而在PMMA颗粒刺激前就加入白细胞介素4将产生更明显的结果[42]。Im等[49]也发现了类似的作用:白细胞介素4抑制了Ti6Al4V合金磨损颗粒刺激人外周血单核细胞产生的肿瘤坏死因子α和白细胞介素6。在颗粒诱导骨溶解的小鼠颅骨模型中,每日向皮下囊注射白细胞介素4,通过CT观察,能够显著降低聚乙烯颗粒导致的骨溶解,且经白细胞介素4处理的实验组抗酒石酸酸性磷酸酶阳性的破骨细胞数量减少,当用诱导型一氧化氮合酶作为M1的标记物,类几丁质酶3样分子3作为M2的标记物时发现聚乙烯颗粒处理组的小鼠有明显的M1/M2比率增高,而继续用白细胞介素4处理后又降低[29]。这些证据表明,白细胞介素4能够减少和抑制M1型巨噬细胞极化或促进M2型巨噬细胞极化。Pajarinen等[50]的研究也指出巨噬细胞对颗粒刺激的反应方式依赖于巨噬细胞的极化状态,诱导巨噬细胞向M2极化可能是限制颗粒诱导炎症的一种手段。也有研究称白细胞介素10能过增强JAK/STAT3信号通路的活性,抑制核因子κB信号通路和JAK/STATl的活性,抑制磨损颗粒刺激极化巨噬细胞向M1型巨噬细胞表型分化,并且可以极化巨噬细胞向M2型巨噬细胞表型转化,甚至可以转换M1型巨噬细胞表型向M2型巨噬细胞表型逆转[51]。最近报道指出姜黄素能够诱导M2型巨噬细胞极化并产生抗炎细胞因子白细胞介素10,调节巨噬细胞极化抑制Ti颗粒引起的炎症反应[52]。综上,目前体内外的研究能够说明白细胞介素4、白细胞介素10及姜黄素等对巨噬细胞极化的调节作用,并可能为一种治疗磨损颗粒引起骨溶解的有效方法。"
| [1] Kurtz S,Ong K,Lau E,et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Bone Joint Surg Am. 2007;89:780-785.[2] Inacio MC,Ake CF,Paxton EW,et al.Sex and risk of hip implant failure:assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173(6):435-441.[3] Marshall A, Ries MD, Paprosky W,et al.How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J Am Acad Orthop Surg. 2008;16 (Suppl 1): S1-S6.[4] Wynn TA,Chawla A,Pollard JW.Macrophage biology in development, homeostasis and disease. Nature. 2013;496 (7446):445-455.[5] Martinez FO,Sica A, Mantovani A,et al.Macrophage activation and polarization. Front Biosci. 2008;13:453-461. [6] Chaudhuri A.Regulation of macrophage polarization by RON receptor tyrosine kinase Signaling.Front Immunol. 2014;5:546.[7] Mazzon C,Zanotti L,Wang L,et al.CCRL2 regulates M1/M2 polarization during EAE recovery phase.J Leukoc Biol. 2016;99(6): 1027-1033.[8] Glass CK,Natoli G.Molecular control of activation and priming in macrophages. Nature Immunol. 2015;17(1):26-33.[9] Nguyen KD,Yifu Q,Xiaojin C,et al.Alternatively activated macrophages produce catecho-amines to sustain adaptive thermogenesis.Nature. 2011;480(7375):104-108.[10] Liu X,Liu J,Zhao S,et al.Interleukin-4 is essential for microglia/ macrophage M2 polarization and long-term recovery after cerebral ischemia.Stroke. 2016;47(2):498-504.[11] Binger KJ,Gebhardt M,Heinig M,et al.High salt reduces the activation of IL-4 and IL-13 stimulated macrophages.J Clin Invest. 2015;125(11):4223-4238.[12] Zhu Y,Tan W,Demetriades AM,et al.IL-17A neutralization alleviated ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization.Immunology. 2016;147(4):414-428.[13] Ilao NB,Lu Mil,Fan YH,et al.Macrophages in tumor microenvironments and the progression of tumors.Clin Dev Immunol. 2012;2012:948098.[14] Buchacher T,Ohradanova-Repic A,Stockinger H,et al.M2 polarization of human macrophages favors survival of the intracellular pathogen chlamydia pneumoniae. PLoS One. 2015; 10(11):e0143593.[15] Khallou-Laschet J,Varthaman A,Fornasa G,et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5(1): e8852.[16] Bourlier V,Zakaroff-Girard A,Miranville A,et al.Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117(6):806-815.[17] Lumeng CN,Bodzin JL,Saltiel AR.Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175-184.[18] Li K,Guo Q,Wang CN,et al.Comparative analysis of phenotypes of classically(M1) and alternatively(M2) activated macrophages.Curr Immunol. 2008;28(3):177-183.[19] Hall CJ,Boyle RH,Astin JW,et al.Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating beta-oxidation-dependent mitochondrial ROS production.Cell Metabol. 2013;18(2):265-278.[20] Gordon S,Martinez FO. Alternative activation of macrophages:mechanism and functions. Immunity. 2010;32: 593-604.[21] Luyendyk JP, Schabbauer GA, Tencati M, et al. Genetic analysis of the role of the P13K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. Immunol. 2008;180(6):4218-4226.[22] Xu F, Kang Y, Zhang H, et al. AKT1-mediated regulation of macrophage polarization in a murine model of Staphylococcus aureus pulmonary infection.Infect Dis. 2013;208(3):528-538.[23] Kono Y,Kawakami S,Higuchi Y,et al.In vitro evaluation of inhibitory effect of nuclear factor -kappaB activity by small interfering RNA on pro-tumor characteristics of M2- like macrophages. Biol Pharm Bull. 2014;37(1):137-144.[24] Hah YS,Cheon YH,Lim HS,et al.Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-kappaB activation.PLoS One. 2014;9(2):e87733.[25] Gadang V,Kohli R,Myronovych A,et al.MLK3 promotes metabolic dysfunction induced by saturated fatty acid-enriched diet.Am J Physiol Endocrinol Metab. 2013;305:E549-556.[26] Ma LJ,Corsa BA, Zhou J,et al.Angiotensin type1 receptor modulates macrophage polarization and renal injury in obesity.Am J Physiol Renal Physiol. 2011;300:F1203-1213.[27] Obstfeld AE,Sugaru E,Thearle M,et al.C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis.Diabetes. 2010;59:916-925.[28] Kang K,Reilly SM,Karabacak V,et al.Adipocyte-derived Th2cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity.Cell Metab. 2008;7:485-495.[29] Rao AJ,Gibon E,Ma T,et al. Revision joint replacement,wear par-ticles,and macrophage polarization.Acta Biomaterialia. 2012; 8(7) :2815-2823.[30] Chen W,Li Z,Guo Y,et al.Wear particles impair antimicrobial activity via suppression of reactive oxygen species generation and ERKL/2 phosphorylation in activated macrophages.Inflammation. 2015;38(3):1289-1296.[31] Purdue E.Alternative macrophage activation in periprosthetic osteolysis.Autoimmunity.2008;41:212-217.[32] Mosser DM, Edwards JP.Exploring the full spectrum of macrophage activation.Nat Rev Immunol. 2008;8:958-969.[33] Takeuch O,Akira S.Epigenetic control of macrophage polarization.Eur J Immunol.2011;41:2490-2493.[34] Ho VW,Sly LM.Derivation and characterization of murine alternatively activated (M2) macrophages. Methods Mol Biol. 2009;531:173-185.[35] Koulouvaris P,Ly K,Ivashkiv LB,et al.Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis.J Orthop Res.2008;26:106-116.[36] Rao AJ, Gibon E, Ma T,et al.Revision joint replacement,wear particles,and macrophage polarization.Acta Biomater.2012;8: 2815-2823.[37] Maitra R,Clement CC,Crisi GM,et al.Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PLoS ONE.2013; 3: e2438.[38] Potnis PA,Dutta DK,Wood SC.Toll-like receptor 4 signaling pathway mediates proinflammatory immune response to cobalt-alloy particles.Cell Immunol.2013;282:53-65.[39] Konttinen YT,Pajarinen J.Adverse reactions to metal-on-metal implants.Nat Rev Rheumatol.2013;(9):5-6.[40] Pearl JI,Ma T,Irani AR,et al.Role of the toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials.2011;32:5535-5542.[41] Joseph KA, Yao ZY. Macrophage polarization in response to wear particles in vitro.Cell Mol Immunol.2013;10(6):471-482.[42] Kim SY,Choi YJ, Joung SM, et al.Hypoxic stress up-regulates the expression of toll-like receptor 4 in macrophages via hypoxia-inducible factor.Immunology.2010;129:516-524.[43] Pajarinen J,Kouri VP,Jämsen E,et al. The response of macrophages to titanium particles is determined by macrophage polarization.Acta Biomater.2013;9:9229-9240.[44] Konttinen YT,Pajarinen J,Takakubo Y, et al.Macrophage polarization and activation in response to implant debris:influence by "particle disease" and "ion disease".J Long Term Eff Med Implants. 2014;24(4):267-281.[45] Murray PJ,Wynn TA.Protective and pathogenic functions of macrophage subsets.Nat Rev Immunol.2011;11:723-737.[46] Jämsen E,Kouri VP,Olkkonen J, et al.Characterization of macrophage polarizing cytokines in the aseptic loosening of total hip replacements.J Orthop Res. 2014;32(9)1241-1246.[47] Jämsen E,Kouri VP, Ainoia M, et al Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors in tissues around aseptically loosened hip implants.J Biomed Mater Res A.2017; 105(2):454-463.[48] Trindade MC,Nakashima Y, Lind M, et al.Interleukin-4 inhibits granulocyte-macrophage colony-stimulating factor, interleukin-6, and tumor necrosis factor-α expression by human monocytes in response to polymethylmethacrylate particle challenge in vitro.J Orthop Res.1999;17(6):797-802.[49] Im GI, Han JD. Suppressive effects of interleukin-4 and interleukin-10 on the production of proinflammatory cytokines induced by titanium-alloy particles. Biomed Mater Res. 2001;58: 531-536.[50] Pajarinen J,Kouri VP,Jamsen E,et al.The response of macrophages to titanium particles is determined by macrophage polarization.Acta Biomater.2013;9:9229-9240.[51] Jiang J, Jia T, Gong W, et al.Macrophage polarization in IL-10 treatment of particle-induced inflammation and osteolysis. Am J Pathol. 2016;186(1):57-66.[52] Li B, Hu Y, Zhao Y, et al. Curcumin attenuates titanium particle-induced inflammation by regulating macrophage polarization in vitro and in vivo. Front Immunol. 2017;8:55. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [3] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [4] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [5] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [6] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [7] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [8] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [9] | Zhao Zhongyi, Li Yongzhen, Chen Feng, Ji Aiyu. Comparison of total knee arthroplasty and unicompartmental knee arthroplasty in treatment of traumatic osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 854-859. |
| [10] | Liu Shaohua, Zhou Guanming, Chen Xicong, Xiao Keming, Cai Jian, Liu Xiaofang. Influence of anterior cruciate ligament defect on the mid-term outcome of fixed-bearing unicompartmental knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 860-865. |
| [11] | Zhang Nianjun, Chen Ru. Analgesic effect of cocktail therapy combined with femoral nerve block on total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 866-872. |
| [12] | Yuan Jun, Yang Jiafu. Hemostatic effect of topical tranexamic acid infiltration in cementless total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 873-877. |
| [13] | Li Yan, Wang Pei, Deng Donghuan, Yan Wei, Li Lei, Jiang Hongjiang. Electroacupuncture for pain control after total knee arthroplasty: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 957-963. |
| [14] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [15] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||