Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (10): 1558-1564.doi: 10.3969/j.issn.2095-4344.2017.10.014
Previous Articles Next Articles
Influence of collagen coating on the biocompatibility of three-dimensional printed implants
Li Sai-na 1, 2, Kang Ji-yao2, Gao Jian-ping2, Gao Yi3, Luo Yuan-ming4, Zhang Gui-feng2, Wang Ming-lin1
- 1 College of Food Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong Province, China; 2 National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China; 3 Zhujiang Hospital of Southern Medical University, Guangzhou 510280, Guangdong Province, China; 4 Institute of Microbiology, Chinese Academy of Sciences, Beijing 100190, China
-
Received:2016-11-13Online:2017-04-08Published:2017-05-08 -
Contact:Zhang Gui-feng, M.D., Researcher, Doctoral supervisor, National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China -
About author:Li Sai-na, College of Food Science and Engineering, Shandong Agricultural University, Tai’an 271018, Shandong Province, China; National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China -
Supported by:the Natural Science Foundation of Guangdong Province, No. 2014A030312013; the National High-Tech Research and Development Program of China (863 Program), No. 2014AA022109
CLC Number:
Cite this article
Li Sai-na, Kang Ji-yao, Gao Jian-ping, Gao Yi, Luo Yuan-ming, Zhang Gui-feng, Wang Ming-lin. Influence of collagen coating on the biocompatibility of three-dimensional printed implants[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(10): 1558-1564.
share this article
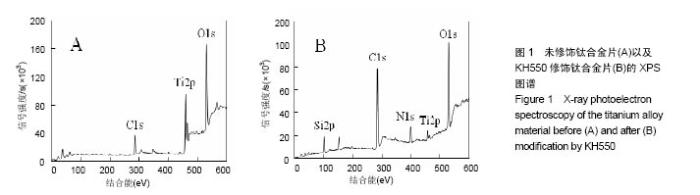
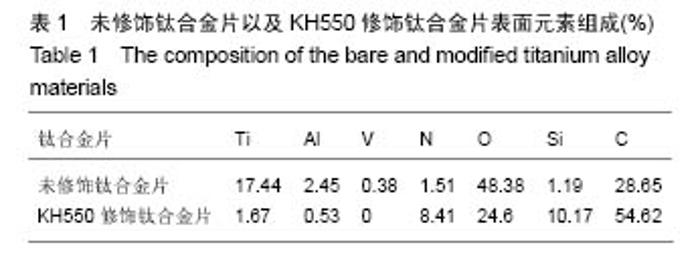
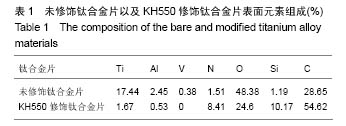
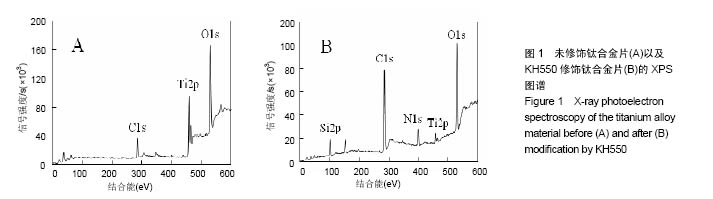
2.1 KH550修饰钛合金片XPS分析结果 采用XPS分析氨基硅烷修饰前后样品表面元素组成的变化。图1是未经修饰钛合金片(经Piranha溶液处理)以及经KH550修饰后钛合金片的XPS扫描图谱,表1列出了每种样品表面Ti、Al、V、N、O、Si、C几种元素的比例。理论上未修饰钛合金片表面没有氮元素,实验检测出的未修饰钛合金片表面1.51%的N属非特异性吸附空气中的N2。KH-550修饰的钛合金片XPS图谱中出现N1s信号峰,其结合能为399.4 eV,与-NH2中N1s结合能对应,表明钛合金片经氨基硅烷修饰后其表面存在N元素;修饰后C1s信号峰出现在结合能284.8 eV,与KH550中的C-C键对应,证明经修饰后KH550成功偶联在钛合金片表面;Si2p信号峰在102.2 eV出现,说明在Si-OH与KH550之间形成了Si-O键。"
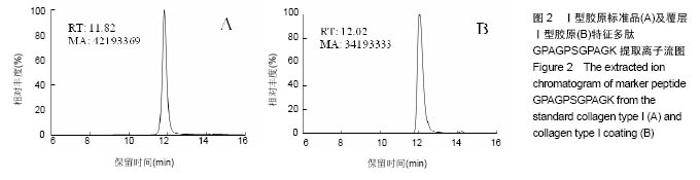
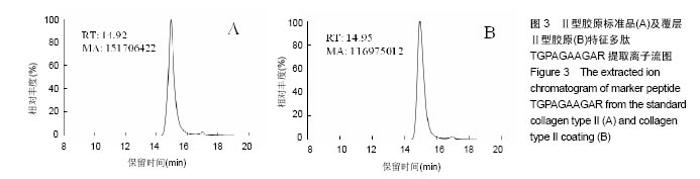
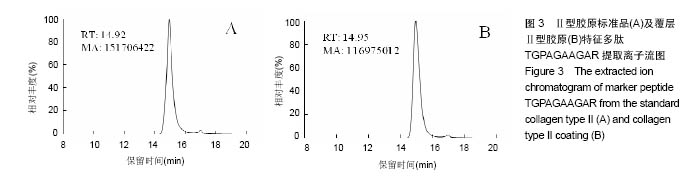
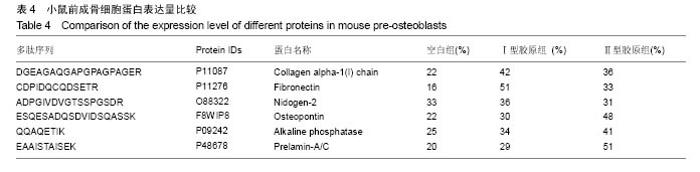
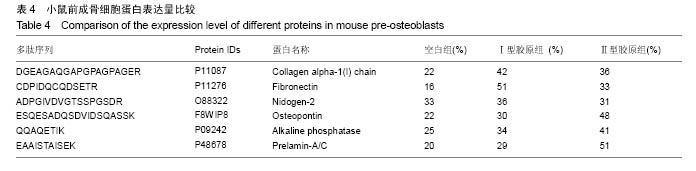
2.2 胶原覆层钛合金片HPLC-MS分析 将酶解后的Ⅰ型覆层胶原及Ⅰ型胶原标准品用HPLC-MS方法对其进行全扫描分析。以全部牛Ⅰ型胶原蛋白为数据库,将酶解多肽信息用SEQUEST软件进行数据库搜索,筛选出在两组样品中均具有阳性结果[27],较高信号值的Ⅰ型胶原特征多肽为GPAGPSGPAGK。以两组样品中该特征多肽的提取离子流图积分峰面积(图2),得到两组样品中GPAGPSGPAGK特征肽段的含量比例为0.81︰1。以同样的方法得到Ⅱ型覆层胶原及Ⅱ型胶原标准品中特征多肽TGPAGAAGAR的含量比例为0.77︰1(图3)。已知胶原标准品质量浓度均为 1 g/L,则钛合金片(Φ10 cm)上的Ⅰ型胶原覆层量为 0.81 mg,Ⅱ型胶原覆层量为0.77 mg。"
| [1]de Andrade DP, de Vasconcellos LM, Carvalho IC, et al. Titanium-35niobium alloy as a potential material for biomedical implants: In vitro study. Mater Sci Eng C Mater Biol Appl. 2015;56:538-544.[2]Kolmas J, Oledzka E, Sobczak M, et al. Nanocrystalline hydroxyapatite doped with selenium oxyanions: a new material for potential biomedical applications.Mater Sci Eng C Mater Biol Appl. 2014;39:134-142.[3]Joo JY, Amin ML, Rajangam T, et al. Fibrinogen as a promising material for various biomedical applications. Molecular & Cellular Toxicology. 2015; 11(1):1-9.[4]Mosadegh B, Xiong G, Dunham S, et al. Current progress in 3D printing for cardiovascular tissue engineering. Biomed Mater. 2015;10(3):034002.[5]Farnoush H, Aghazadeh Mohandesi J, Çimeno?lu H. Micro-scratch and corrosion behavior of functionally graded HA-TiO2 nanostructured composite coatings fabricated by electrophoretic deposition. J Mech Behav Biomed Mater. 2015;46:31-40.[6]Himics L, Tóth S, Veres M, et al. Effective implantation of light emitting centers by plasma immersion ion implantation and focused ion beam methods into nanosized diamond. Applied Surface Science. 2015; 328:577-582.[7]Quan Z, Ni E, Ogasawara Y, et al. Nano-size multiple metal oxide anode electrodes synthesized from layered double hydroxides - Electrochemical reaction mechanism and surface morphology change during reaction with lithium ion. Solid State Ionics. 2014; 268: 268-272. [8]Liang CY, Zhong X, Wang HS, et al. Femtosecond laser induced micropatterns and in-situ deposition of Ca/P phase and collagen on Ti surface. Materials Chemistry and Physics. 2015; 158: 115-120.[9]Hauser J, Koeller M, Bensch S, et al. Plasma mediated collagen-I-coating of metal implant materials to improve biocompatibility. J Biomed Mater Res A. 2010;94(1):19-26.[10]Schulz MC, Korn P, Stadlinger B, et al. Coating with artificial matrices from collagen and sulfated hyaluronan influences the osseointegration of dental implants.J Mater Sci Mater Med. 2014;25(1):247-258.[11]Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris). 2005;53(7):430-442.[12]Huang CY, Kuo JM, Wu SJ, et al. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion-hydro-extraction process. Food Chem. 2016;190:997-1006.[13]Martínezortiz MA, Hernándezfuentes AD, Pimentelgonzález DJ, et al. Extraction and characterization of collagen from rabbit skin: partial characterization. CyTA - Journal of Food. 2015; 13(2):253-258.[14]Shanmugam G, Reddy SMM, Madhan B,et al. Method of addition of acetonitrile influences the structure and stability of collagen. Process Biochemistry. 2014;49(2): 210-216.[15]Li GY, Fukunaga S, Takenouchi K, et al. Comparative study of the physiological properties of collagen, gelatin and collagen hydrolysate as cosmetic materials. Int J Cosmet Sci. 2005; 27(2):101-106.[16]Hauser J, Ring A, Schaffran A, et al. In vivo analysis of tissue response to plasma-treated collagen-I-coated titanium alloys. Eur Surg Res. 2009;43(3):262-268.[17]Felgueiras HP, Sommerfeld SD, Murthy NS, et al. Poly(NaSS) functionalization modulates the conformation of fibronectin and collagen type I to enhance osteoblastic cell attachment onto Ti6Al4V. Langmuir. 2014;30(31):9477-9483.[18]Gigante A, Bevilacqua C, Cappella M, et al. Engineered articular cartilage: influence of the scaffold on cell phenotype and proliferation. J Mater Sci Mater Med. 2003;14(8): 713-716.[19]Buma P, Pieper JS, van Tienen T, et al. Cross-linked type I and type II collagenous matrices for the repair of full-thickness articular cartilage defects--a study in rabbits. Biomaterials. 2003;24(19):3255-3263.[20]Nehrer S, Breinan HA, Ramappa A, et al. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18(11):769-776.[21]Su PJ, Chen WL, Li TH, et al. The discrimination of type I and type II collagen and the label-free imaging of engineered cartilage tissue. Biomaterials. 2010;31(36):9415-9421.[22]Chang KY, Hung LH, Chu IM, et al. The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J Biomed Mater Res A. 2010; 92(2):712-723. [23]Cao YL, Liu T, Pang J, et al. Glucan HBP-A increase type II collagen expression of chondrocytes in vitro and tissue engineered cartilage in vivo. Chin J Integr Med. 2015;21(3): 196-203.[24]Kontturi LS, Järvinen E, Muhonen V, et al. An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv Transl Res. 2014;4(2):149-158.[25]Yao Y, Ma YZ, Qin M, et al. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids Surf B Biointerfaces. 2008;66(2): 233-239.[26]孙爱梅,张贵锋,倪文,等. 胶原蛋白降解物高效液相色谱/质谱联用分析[J]. 中国生物工程杂志, 2005, 25(2): 66-72.[27]张贵锋,刘涛,王前,等. 高效液相色谱/质谱法识别不同明胶酶解产物中特征多肽[J]. 分析化学, 2008, 36(11): 1499-1504.[28]Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.[29]Shah FA, Trobos M, Thomsen P, et al. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants - Is one truly better than the other. Mater Sci Eng C Mater Biol Appl. 2016;62:960-966.[30]Crespo L, Hierro-Oliva M, Barriuso S, et al. On the interactions of human bone cells with Ti6Al4V thermally oxidized by means of laser shock processing. Biomed Mater. 2016;11(1):015009.[31]Calzado-Martín A, Crespo L, Saldaña L,et al. Human bone-lineage cell responses to anisotropic Ti6Al4V surfaces are dependent on their maturation state. J Biomed Mater Res A. 2014;102(9):3154-3166.[32]Barão VA, Mathew MT, Assunção WG, et al. Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH - an electrochemical study. Clin Oral Implants Res. 2012; 23(9):1055-1062.[33]Mohseni E, Zalnezhad E, Bushroa AR.Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. International Journal of Adhesion & Adhesives. 2014;48(1):238-257.[34]Durdu S, Deniz OF, Kutbay I, et al.Characterization and formation of hydroxyapatite on Ti6Al4V coated by plasma electrolytic oxidation. Journal of Alloys and Compounds. 2013; 551: 422-429.[35]Arifin A, Sulong AB, Muhamad N, et al. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: a review. Materials & Design. 2014; 55(6): 165-175. [36]Benea L, Mardare-Danaila E, Mardare M, et al. Preparation of titanium oxide and hydroxyapatite on Ti–6Al–4V alloy surface and electrochemical behaviour in bio-simulated fluid solution. Corrosion Science. 2014; 80(3): 331-338.[37]Liu Y, Cui H, Zhuang X, et al. Nano-hydroxyapatite surfaces grafted with electroactive aniline tetramers for bone-tissue engineering. Macromol Biosci. 2013;13(3):356-365.[38]Raghavan RN, Muthukumar T, Somanathan N, et al. Biomimetic mineralization of novel silane crosslinked collagen. Mater Sci Eng C Mater Biol Appl. 2013;33(4):1983-1988.[39]Zhu H, Hu C, Zhang F, et al. Preparation and antibacterial property of silver-containing mesoporous 58S bioactive glass. Mater Sci Eng C Mater Biol Appl. 2014;42:22-30.[40]Chiu CK, Ferreira J, Luo TJ, et al. Direct scaffolding of biomimetic hydroxyapatite-gelatin nanocomposites using aminosilane cross-linker for bone regeneration. J Mater Sci Mater Med. 2012;23(9):2115-2126.[41]张杭州, 孙羽, 王琳, 等. 羟基磷灰石/TiO2纳米管复合物的生物相容性[J]. 中国组织工程研究, 2014, 18(3): 335-340.[42]鲁雄, 冯波, 翁杰, 等. 生物材料表面微纳结构对成骨相关细胞的影响[J]. 中国材料进展, 2013, 32(10): 612-622.[43]Higuchi A, Ling QD, Chang Y, et al. Physical cues of biomaterials guide stem cell differentiation fate. Chem Rev. 2013;113(5):3297-3328.[44]Lee TT, García JR, Paez JI, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14(3):352-360.[45]Shafiq M, Jung Y, Kim SH. Insight on stem cell preconditioning and instructive biomaterials to enhance cell adhesion, retention, and engraftment for tissue repair. Biomaterials. 2016;90:85-115. [46]Spicer V, Ezzati P, Neustaeter H, et al. 3D HPLC-MS with Reversed-Phase Separation Functionality in All Three Dimensions for Large-Scale Bottom-Up Proteomics and Peptide Retention Data Collection. Anal Chem. 2016;88(5):2847-5285.[47]Tscheliessnig AL, Konrath J, Bates R, et al. Host cell protein analysis in therapeutic protein bioprocessing - methods and applications. Biotechnol J. 2013;8(6):655-670.[48]Hou J, Tobe BT, Lo F, et al. Combined total proteomic and phosphoproteomic analysis of human pluripotent stem cells. Methods Mol Biol. 2013;1029:163-189.[49]Köcher T, Pichler P, Swart R, et al. Analysis of protein mixtures from whole-cell extracts by single-run nanoLC-MS/MS using ultralong gradients. Nat Protoc. 2012;7(5):882-890. |
| [1] | Xue Yadong, Zhou Xinshe, Pei Lijia, Meng Fanyu, Li Jian, Wang Jinzi . Reconstruction of Paprosky III type acetabular defect by autogenous iliac bone block combined with titanium plate: providing a strong initial fixation for the prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1424-1428. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [4] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [5] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [6] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [7] | Hu Weifan, Zheng Li, Li Dadi, Sun Yang, Zhao Fengchao. Overexpression of miR-25 downregulates titanium particle-induced osteoclast differentiation through the NFATc1 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 682-687. |
| [8] | Qiu Peng, Fu Qilin, Liu Min, Lan Yuyan, Wang Pin. Comparison of oral micro-adhesion on polyetheretherketone, zirconium dioxide, and pure titanium abutment [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 540-545. |
| [9] | Chen Shuo, Xiao Dongqin, Li Xingping, Ran Bin, Shi Feng, Zhang Chengdong, Deng Li, Huang Nanxiang, Liu Kang, Feng Gang, Duan Ke. Preparation and characterization of tantalum functional coating on titanium implant [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 546-552. |
| [10] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [11] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [12] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [13] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [14] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [15] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||