Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (5): 1139-1146.doi: 10.12307/2026.041
Previous Articles Next Articles
Mechanism of depression-like behavior in chronic social defeat stress mice based on high-throughput sequencing
Zhang Di1, Zhao Jun2, Ma Guangyue1, Sun Hui1, Jiang Rong1
- 1Department of Basic Medical Sciences, 2Department of Nursing (School of Gerontology), Binzhou Medical University, Yantai 264003, Shandong Province, China
-
Received:2024-12-02Accepted:2025-02-12Online:2026-02-18Published:2025-06-24 -
Contact:Sun Hui, PhD, Professor, Master’s supervisor, Department of Basic Medical Sciences, Binzhou Medical University, Yantai 264003, Shandong Province, China Co-corresponding author: Jiang Rong, PhD, Associate professor, Master’s supervisor, Department of Basic Medical Sciences, Binzhou Medical University, Yantai 264003, Shandong Province, China -
About author:Zhang Di, MS candidate, Department of Basic Medical Sciences, Binzhou Medical University, Yantai 264003, Shandong Province, China -
Supported by:National Natural Science Foundation of China, Nos. 82301726 (to JR) and 81971281 (to SH); Natural Science Foundation of Shandong Province, No. ZR2022QH087 (to JR)
CLC Number:
Cite this article
Zhang Di, Zhao Jun, Ma Guangyue, Sun Hui, Jiang Rong. Mechanism of depression-like behavior in chronic social defeat stress mice based on high-throughput sequencing[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1139-1146.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
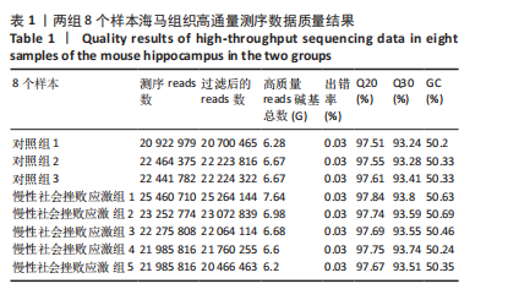
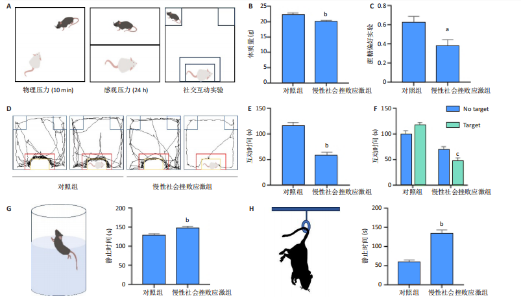
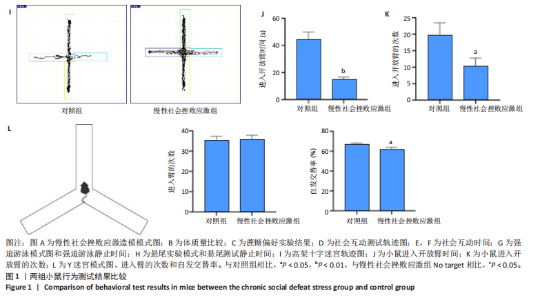
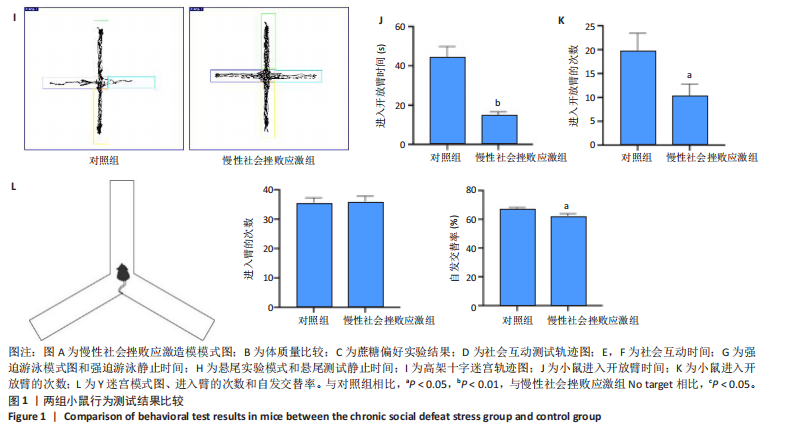
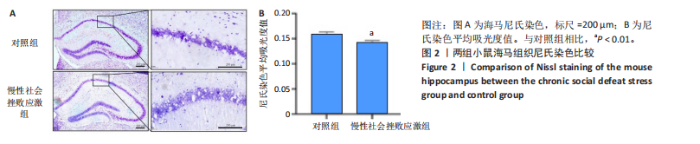
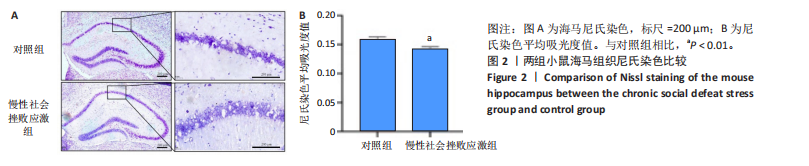
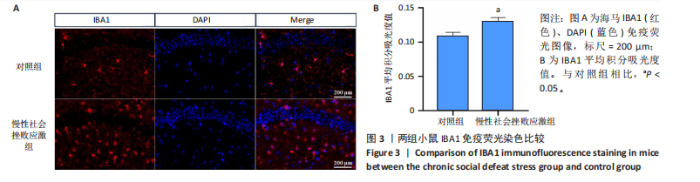
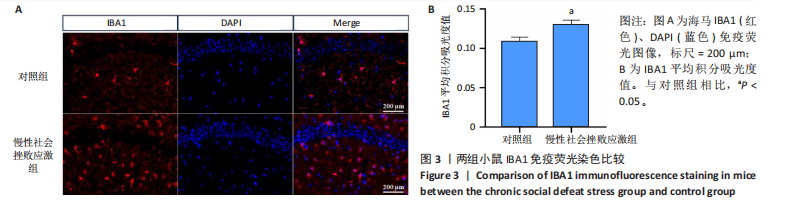
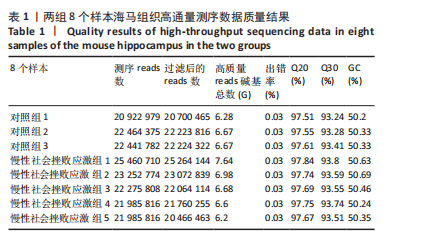
2.1 实验动物数量分析 实验选用小鼠30只,对照组小鼠10只,模型组20只小鼠造模过程中死亡2只,随机选取慢性社会挫败应激小鼠造模成功11只,进入结果分析21只小鼠。 2.2 慢性社会挫败应激小鼠表现出焦虑、认知受损和抑郁样行为 2.2.1 慢性社会挫败应激诱导小鼠抑郁模型 造模流程图见图1A。与对照组相比,慢性社会挫败应激组小鼠的体质量明显减轻(P < 0.01),见图1B。 2.2.2 蔗糖偏好测试结果 与对照组相比慢性社会挫败应激组小鼠蔗糖消耗的百分比显著降低 (P < 0.05),见图1C。 2.2.3 社交互动实验结果 慢性社会挫败应激组小鼠社交时间明显减少(P < 0.05或P < 0.01),见图1D-F。 2.2.4 强迫游泳测试和悬尾实验结果 慢性社会挫败应激组小鼠表现出更多的不动时间 (P < 0.01) ,见图1G,H。 2.2.5 高架十字迷宫实验结果 与对照组相比,慢性社会挫败应激组小鼠减少了在开臂上停留时间和进入开臂的次数(P < 0.05或P < 0.01)。这些反应表明了慢性社会挫败应激小鼠出现焦虑行为,见图1I-K。慢性社会挫败应激组小鼠并没有改变进入臂的次数,但减少了探索的自发交替率(P < 0.05),见图1L。 2.3 慢性社会挫败应激小鼠海马脑区神经元受损 尼氏染色法通过尼氏体观察神经元的损伤情况[16]。海马组织神经细胞尼氏体染色结果显示,与对照组相比慢性社会挫败应激组海马神经元染色变浅,出现明显的尼氏体核固缩,严重的还呈现明显的空泡样病变,尼氏体累积吸光度值明显降低(P < 0.01),见图2。 2.4 慢性社会挫败应激诱导小鼠海马脑区炎症反应 IBA1免疫荧光结果显示,慢性社会挫败应激小鼠海马脑区小胶质细胞活化、数量增多(P < 0.05),并且细胞突起消失,呈现阿米巴样的形态,见图3。 2.5 高通量测序数据质量结果 8个样本的测序结果分别得到6.28 G、6.67 G、6.67 G、7.64 G、6.98 G、6.68 G、6.6 G和6.2 G高质量序列,且测序错误率均为0.03%,碱基质量值Q20≥97.55%,Q30≥93.24%,GC含量分布≥50.2%,完全符合预定测序要求,均可用于进一步分析,见表1。"
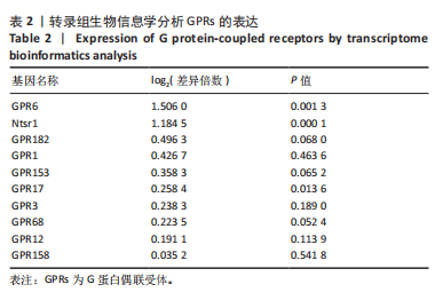
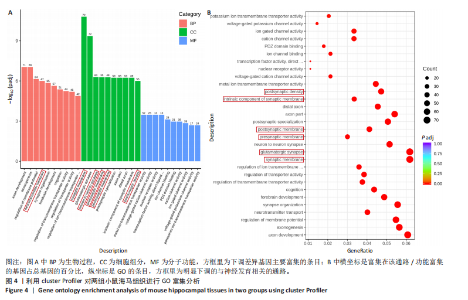
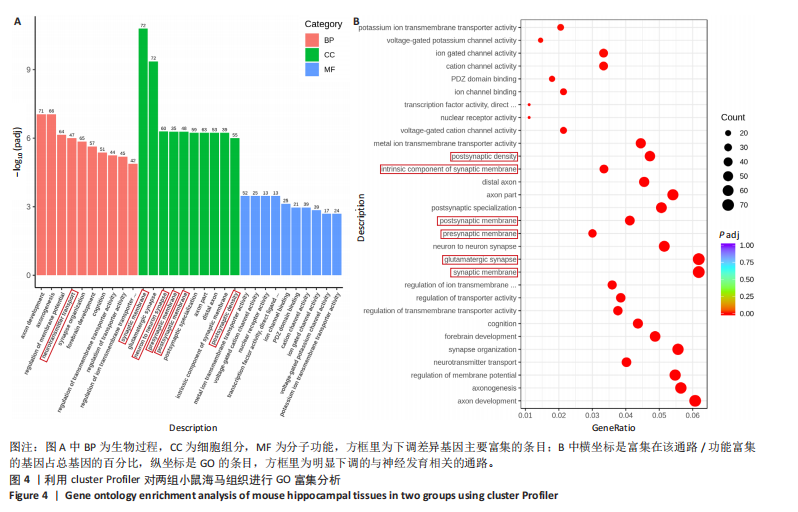
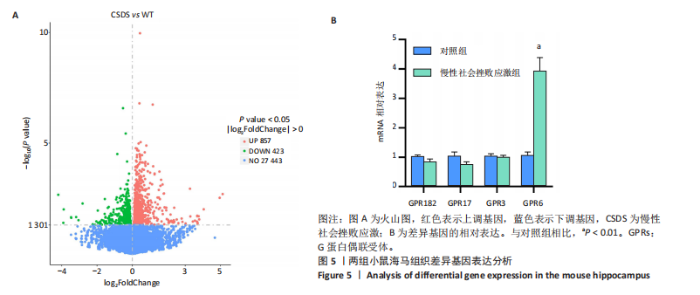
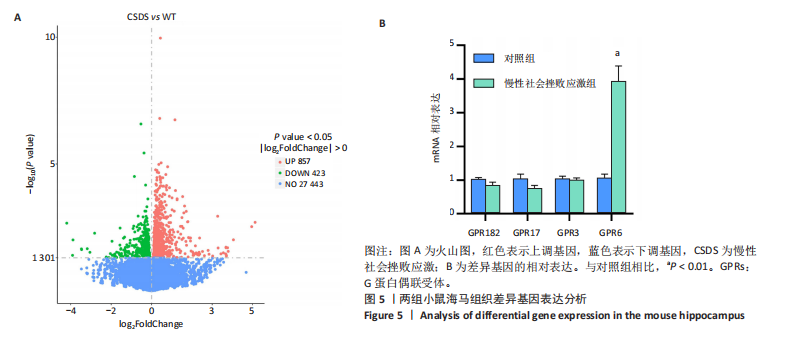
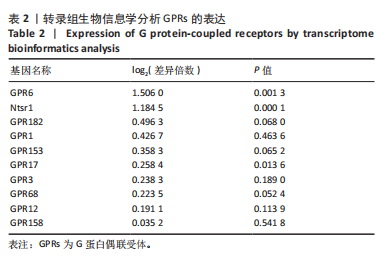
2.6 GO富集分析 为了研究慢性社会挫败应激后对小鼠海马脑区生物学功能的影响,采用cluster Profiler软件进行GO功能富集分析,从生物过程( biological process,BP)、细胞组成( cellular component,CC)和分子功能( molecular function,MF) 类别中分别选取了富集显著性最高的前10个条目结果显示:海马中下调差异基因主要富集在细胞组成的突触结构、突触膜突触后致密区、突触传递、轴突发育等条目,见图4A。这些发现强调慢性社会挫败应激诱导在神经元生长发育中的重要作用。与此同时,神经营养因子信号转导、轴突引导通路等与神经发育相关的通路也明显下调,见图4B。 2.7 差异基因表达分析 比较对照组小鼠和慢性社会挫败应激小鼠海马组织表达谱共发现1 280个差异基因,其中857个基因表达上调,423个基因表达下调。差异基因分布的火山图如图5A所示,其中G蛋白偶联受体是一大类膜蛋白受体的统称,参与多种生理活动,RNA-seq结果显示,应激导致GPRs的表达水平明显上调,见表2,这意味着GPRs可能参与情绪调节行为改变。为了进一步探究慢性社会挫败应激小鼠出现行为学的机制,使用qPCR技术观察慢性社会挫败应激小鼠海马脑区GPRs的表达变化。结果显示,慢性社会挫败应激诱导海马脑区GPR6的表达明显升高(P < 0.01),见图5B。"
| [1] ALVINA K, JODEIRI FM, MONDAL AK. Long term effects of stress on hippocampal function: Emphasis on early life stress paradigms and potential involvement of neuropeptide Y. J Neurosci Res. 2021;99(1): 57-66. [2] VIDEBECH P, RAVNKILDE B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11): 1957-1966. [3] TARTT AN, MARIANI MB, HEN R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699. [4] FRIES GR, SALDANA VA, FINNSTEIN J, et al. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry. 2023;28(1):284-297. [5] DUMAN RS, AGHAJANIAN GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72. [6] 王舒婷,熊欣欣,刘媛媛.细胞骨架调控大脑皮层神经元迁移机制的研究进展[J].华中科技大学学报(医学版),2022,51(2):281-286. [7] PAREKH PK, JOHNSON SB, LISTON C. Synaptic Mechanisms Regulating Mood State Transitions in Depression. Annu Rev Neurosci. 2022;45: 581-601. [8] MCNALLY L, BHAGWAGAR Z, HANNESTAD J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13(6): 501-510. [9] 胡月,曾常茜.小胶质细胞活化与神经炎症相关信号通路的研究进展[J].医学综述,2021,27(20):4005-4010. [10] SUTTON LP, ORLANDI C, SONG C, et al. Orphan receptor GPR158 controls stress-induced depression. Elife. 2018;7:e33273. [11] LABOUTE T, ZUCCA S, HOLCOMB M, et al. Orphan receptor GPR158 serves as a metabotropic glycine receptor: mGlyR. Science. 2023; 379(6639):1352-1358. [12] MLYNIEC K, SIODLAK D, DOBOSZEWSKA U, et al. GPCR oligomerization as a target for antidepressants: Focus on GPR39. Pharmacol Ther. 2021;225:107842. [13] JIRKOF P, BRATCHER N, MEDINA L, et al. The effect of group size, age and handling frequency on inter-male aggression in CD 1 mice. Sci Rep. 2020;10(1):2253. [14] LU Q, MOURI A, YANG Y, et al. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav Brain Res. 2019;372:112053. [15] MARIANI N, CATTANE N, PARIANTE C, et al. Gene expression studies in Depression development and treatment: an overview of the underlying molecular mechanisms and biological processes to identify biomarkers. Transl Psychiatry. 2021;11(1):354. [16] BROSCH K, STEIN F, SCHMITT S, et al. Reduced hippocampal gray matter volume is a common feature of patients with major depression, bipolar disorder, and schizophrenia spectrum disorders. Mol Psychiatry. 2022;27(10):4234-4243. [17] RODDY DW, FARRELL C, DOOLIN K, et al. The Hippocampus in Depression: More Than the Sum of Its Parts? Advanced Hippocampal Substructure Segmentation in Depression. Biol Psychiatry. 2019;85(6):487-497. [18] FRIES GR, SALDANA VA, FINNSTEIN J, et al. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry. 2023;28(1):284-297. [19] APWEILER M, SALIBA SW, SUN L, et al. Modulation of neuroinflammation and oxidative stress by targeting GPR55 - new approaches in the treatment of psychiatric disorders. Mol Psychiatry. 2024;29(12):3779-3788. [20] CABRAL-MARQUES O, MOLL G, CATAR R, et al. Autoantibodies targeting G protein-coupled receptors: An evolving history in autoimmunity. Report of the 4th international symposium. Autoimmun Rev. 2023;22(5):103310. [21] TANAKA S, ISHII K, KASAI K, et al. Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth.J Biol Chem. 2007;282(14):10506-10515. [22] WEI Z, XU X, FANG Y, et al. Rab43 GTPase directs postsynaptic trafficking and neuron-specific sorting of G protein-coupled receptors.J Biol Chem. 2021;296:100517. [23] BRAUNE M, SCHERF N, HEINE C, et al. Involvement of GPR17 in Neuronal Fibre Outgrowth. Int J Mol Sci. 2021;22(21):11683. [24] A RH, GONG Q, TUO YJ, et al. Syringa oblata Lindl extract alleviated corticosterone-induced depression via the cAMP/PKA-CREB-BDNF pathway. J Ethnopharmacol. 2025;341:119274. [25] SINHA K, KUMAWAT A, JANG H, et al. Molecular mechanism of regulation of RhoA GTPase by phosphorylation of RhoGDI. Biophys J. 2024;123(1):57-67. [26] ASLAM M. cAMP/PKA-mediated in vitro angiogenesis: A novel interplay between PKA, RhoA/ROCK, and VEGFR2 signalling. Eur Heart J. 2024; 45(Supplement_1). doi: 10.1093/eurheartj/ehae666.3836 [27] TAN D, ZHANG H, DENG J, et al. RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and p60-Katanin. Cells. 2020;9(1):230. [28] SHIU FH, WONG JC, YAMAMOTO T, et al. Mice lacking full length Adgrb1 (Bai1) exhibit social deficits, increased seizure susceptibility, and altered brain development. Exp Neurol. 2022;351:113994. [29] ZHU S, HU X, BENNETT S, et al. Molecular Structure, Expression and Role of TAFA4 and its Receptor FPR1 in the Spinal Cord. Front Cell Dev Biol. 2022;10:911414. [30] WANG S, DELEON C, SUN W, et al. Alternative splicing of latrophilin-3 controls synapse formation. Nature. 2024;626(7997):128-135. |
| [1] | Haonan Yang, Zhengwei Yuan, Junpeng Xu, Zhiqi Mao, Jianning Zhang. Preliminary study on the mechanisms and efficacy of deep brain stimulation in treating depression [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-9. |
| [2] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [3] | Zhang Jiuxuan, Zhang Jinnan, Sui Xiaofan, Pei Xiaxia, Wei Jianhong, Su Qiang, Li Tian. Effects of ammonia poisoning on cognitive behavior and hippocampal synaptic damage in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1122-1128. |
| [4] | Li Haojing, Wang Xin, Song Chenglin, Zhang Shengnan, Chen Yunxin. Therapeutic efficacy of extracorporeal shock wave therapy in the upper trapezius muscle area combined with exercise control training in patients with chronic non-specific neck pain [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1162-1170. |
| [5] | Liu Yu, Lei Senlin, Zhou Jintao, Liu Hui, Li Xianhui. Mechanisms by which aerobic and resistance exercises improve obesity-related cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1171-1183. |
| [6] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [7] | Leng Xiaoxuan, Zhao Yuxin, Liu Xihua. Effects of different neuromodulatory stimulation modalities on non-motor symptoms in Parkinson’s patients: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1282-1293. |
| [8] | Wen Xiaolong, Weng Xiquan, Feng Yao, Cao Wenyan, Liu Yuqian, Wang Haitao. Effects of inflammation on serum hepcidin and iron metabolism related parameters in patients with type 2 diabetes mellitus: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1294-1301. |
| [9] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [10] | Ma Hong, Ding Xueling, Wang Qi, Lyu Hui, Asya Albusm, Cheng Xinyi, Ma Xiang. Expression and significance of tumor necrosis factor alpha, nuclear factor kappaB and ionized calcium binding adaptor molecule-1 in the hippocampus of mice with aortic dissection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 858-863. |
| [11] | Zhang Tingting, Li Yalong, Yue Haodi, Li Yanjun, Geng Xiwen, Zhang Yuwei, Liu Xiaozhuan. Protection of exosomes derived from bone marrow mesenchymal stem cells of different mouse ages on radiation-induced lung injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 1-9. |
| [12] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [13] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [14] |
Li Tian, Ren Yuhua, Gao Yanping, Su Qiang.
Mechanism of agomelatine alleviating anxiety- and depression-like behaviors in APP/PS1 transgenic mice #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1176-1182.
|
| [15] | Bai Jing, Zhang Xue, Ren Yan, Li Yuehui, Tian Xiaoyu. Effect of lncRNA-TNFRSF13C on hypoxia-inducible factor 1alpha in periodontal cells by modulation of #br# miR-1246 #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 928-935. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||