Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5629-5638.doi: 10.12307/2026.140
U-shaped association between magnesium intake and all-cause and cancer mortality in patients with osteoarthritis
Zhou Haidong1, Lu Yaohong1, Fan Shaoyong2
- 1Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; 2Hongdu Hospital of Traditional Chinese Medicine Affiliated to Jiangxi University of Chinese Medicine, Nanchang 330038, Jiangxi Province, China
-
Received:2025-03-14Accepted:2025-08-01Online:2026-08-08Published:2025-12-25 -
Contact:Fan Shaoyong, MD, Chief physician, Hongdu Hospital of Traditional Chinese Medicine, Nanchang 330038, Jiangxi Province, China -
About author:Zhou Haidong, PhD candidate, Physician, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China -
Supported by:the Jiangxi Provincial Key Research and Development Program Project, No. 20202BBG73028 (to FSY); 2024 Jiangxi Provincial University-level Student Innovation and Entrepreneurship Training Program Project, No. 202410412281 (to ZHD); 2024 Jiangxi Provincial Postgraduate Innovation Special Funds Project, No. YC2024-B239 (to ZHD)
CLC Number:
Cite this article
Zhou Haidong, Lu Yaohong, Fan Shaoyong. U-shaped association between magnesium intake and all-cause and cancer mortality in patients with osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5629-5638.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
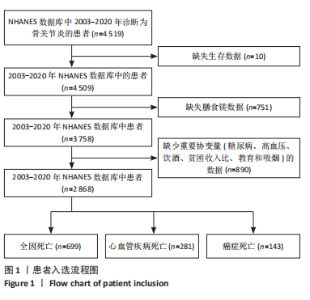
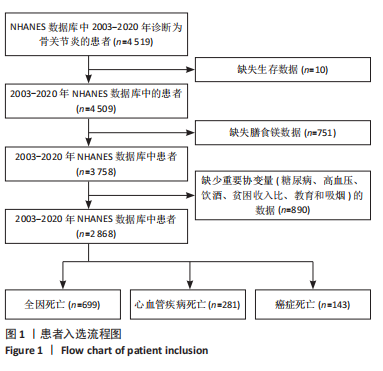
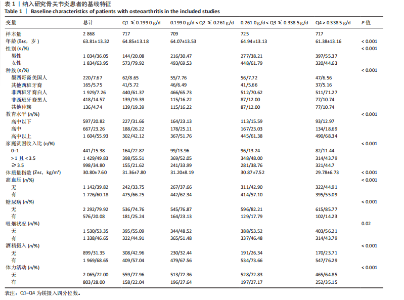
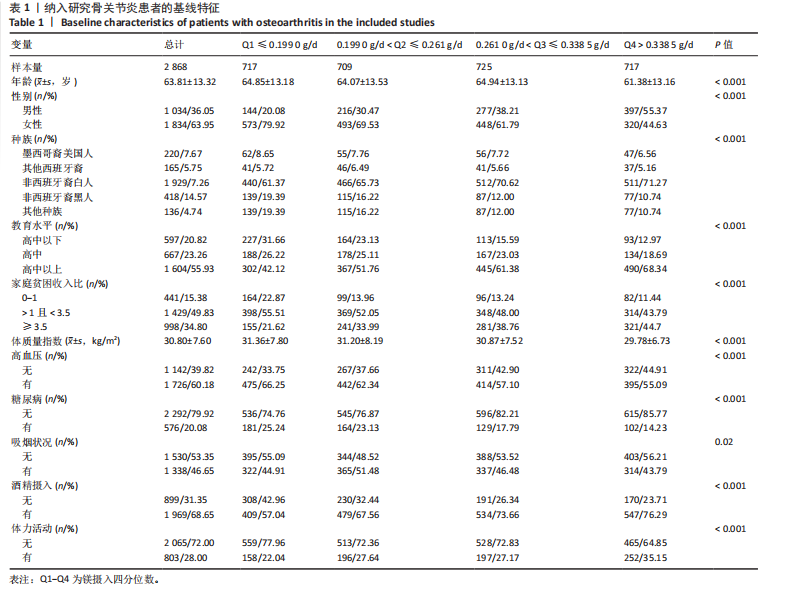
2.1 骨关节炎患者的基线特征 在2003-2020年NHANES数据库中,共招募了4 519例骨关节炎患者,排除生存数据缺失(n=10)、膳食镁摄入数据缺失(n=751)以及缺少关键协变量的患者(n=890),最终纳入2 868例患者参与分析。随访期间,记录了699例全因死亡、281例心血管疾病相关死亡和143例癌症相关死亡。图1展示了人群筛选流程。患者平均年龄(63.81±13.32)岁,其中63.95%为女性,36.05%为男性。表1总结了按膳食镁摄入量四分位数分层后的基线特征,显示了组间年龄、性别、种族、体质量指数、教育、家庭贫困收入比、吸烟状况、饮酒、体力活动、高血压和糖尿病患病率方面的差异(P < 0.05)。四分位数Q1-Q4的镁摄入量范围分别为:Q1≤0.199 0 g/d,0.199 0 g/d < Q2≤0.261 0 g/d,0.261 0 g/d < Q3≤0.338 5 g/d,Q4 > 0.338 5 g/d。 与最低膳食镁摄入量组相比,膳食镁摄入量较高患者患高血压和糖尿病的风险显著降低(P < 0.05)。"
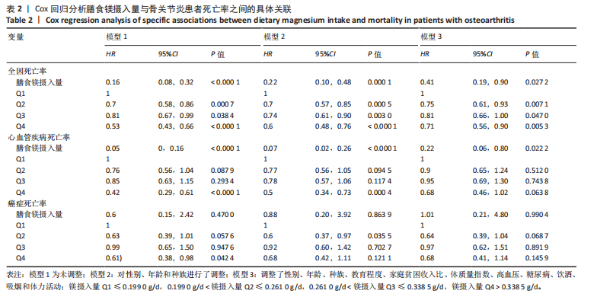
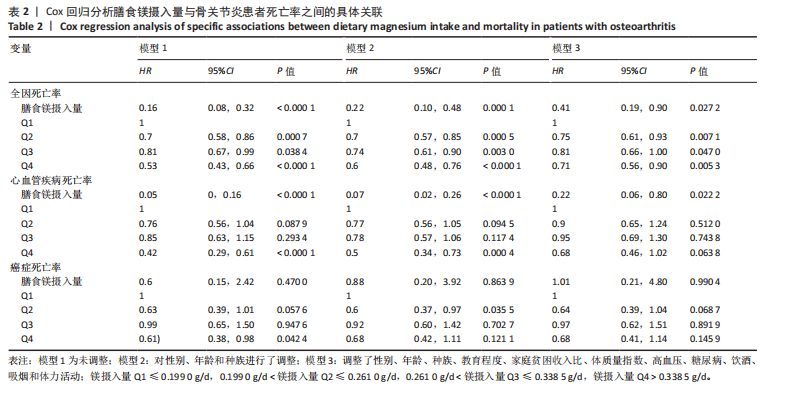
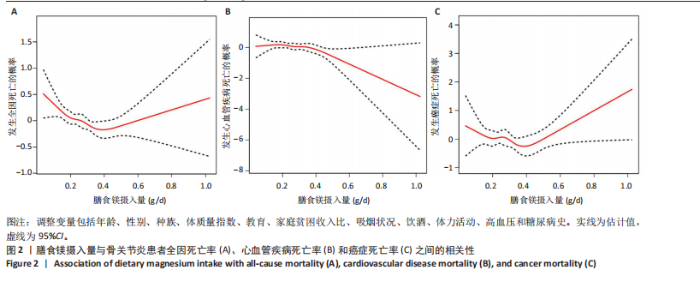
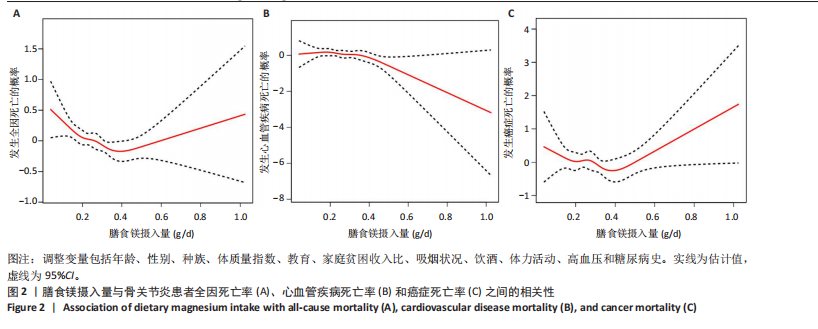
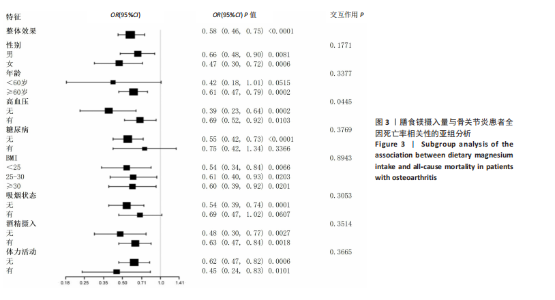
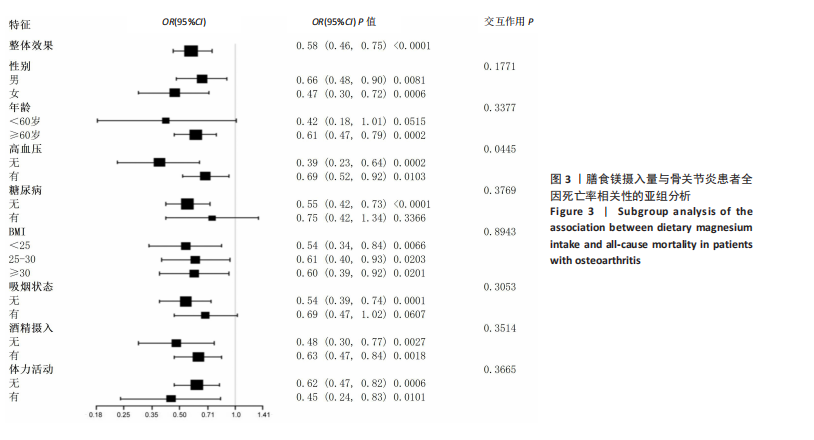
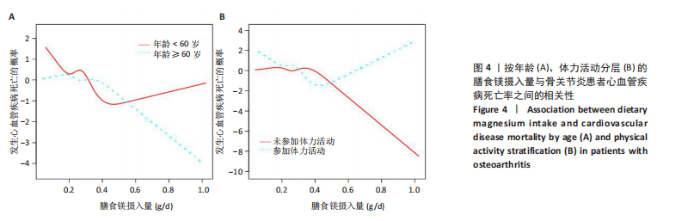
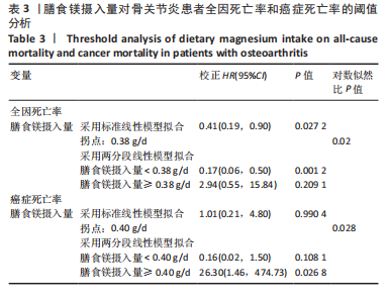
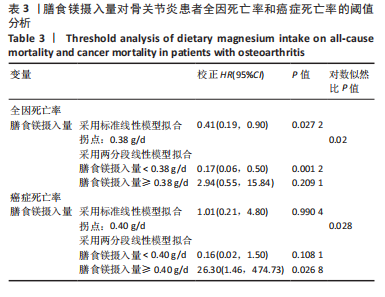
2.2 膳食镁摄入量与死亡率的关系 表2中的Cox回归分析显示了膳食镁摄入量与死亡率之间的具体关联。将膳食镁摄入量作为连续变量纳入分析,结果显示在多变量校正后,较高的膳食镁摄入量显著降低心血管疾病死亡风险,每增加1单位镁摄入量心血管疾病死亡风险下降78%(P=0.022 2),这种显著相关性在全因和癌症死亡率中也有类似的趋势。对于全因死亡(模型3),按膳食镁摄入量四分位数分组的多变量校正结果分别为Q1组[HR=1.00(参考)]、Q2组[HR=0.75,95%CI(0.61,0.93)]、Q3组[HR=0.81,95%CI(0.66,1.00)]、Q4组[HR=0.71,95%CI(0.56,0.90)]。对于心血管疾病死亡率,按膳食镁摄入量四分位数分组的多变量校正结果分别为Q1组[HR=1.00(参考)]、Q2组[HR=0.90,95%CI(0.65,1.24)]、Q3组[HR=0.95,95%CI(0.69,1.30)]。Q4组[HR=0.68,95%CI(0.46,1.02)]。对于癌症死亡率,按膳食镁摄入量四分位数分组的多变量校正结果分别为Q1组[HR=1.00(参考)]、Q2组[HR=0.64,95%CI(0.39,1.04)]、Q3组[HR=0.97, 95%CI(0.62,1.51)]、Q1组[HR=0.68,95%CI(0.41,1.14)]。整体上,与膳食镁摄入量最低组(Q1)相比,膳食镁摄入量较高的骨关节炎患者全因、心血管疾病和癌症死亡率均较低。 2.3 膳食镁摄入量与死亡率之间的剂量反应关系 如图2和表3所示,广义加性模型和平滑曲线拟合进一步阐明了膳食镁摄入量与全因死亡率之间的U形非线性关系(对数似然比P=0.02),并对所有协变量进行了调整。结果显示,当膳食镁摄入量大于阈值0.38 g/d时,镁摄入量增加与骨关节炎患者全因死亡风险增加显著相关,每增加1单位镁摄入量全因死亡风险增加194%[HR=2.94,95%CI(0.55,15.84)];当镁摄入量低于该阈值时,全因死亡风险随镁摄入量增加而下降[HR=0.17,95%CI(0.06,0.50)]。膳食镁摄入量与心血管疾病死亡率呈线性负相关。对于癌症死亡率,也观察到了类似的U形关系(对数似然比P=0.028),其中最低死亡风险镁摄入量阈值为0.40 g/d,在此阈值之前和之后,每增加1单位镁摄入量癌症死亡风险分别变化84%[HR=0.16,95%CI(0.01,1.50)]和2 530%[HR=26.30,95%CI(1.46,474.73)]。 2.4 亚组分析结果 为了评估膳食镁摄入量与全因死亡率的关联是否在人群中保持一致,并识别潜在的高风险人群,按年龄、性别、体质量指数、高血压、糖尿病、体力活动、吸烟状况、饮酒状态等分层的亚组进行分析和交互作用检验。基于最低风险阈值水平对膳食镁摄入量进行二分,相对于膳食镁摄入量水平较低的患者(< 0.38 g/d),膳食镁摄入量较高显示出对骨关节炎患者全因死亡风险的保护作用[HR=0.58,95%CI(0.46,0.75)];膳食镁摄入量与全因死亡率的关联存在显著高血压人群差异(交互作用P=0.044 5),高血压骨关节炎患者相比非高血压患者的生存劣势更为明显;膳食镁摄入量与年龄、体质量指数、性别、糖尿病、体力活动、吸烟状态、饮酒状态之间未观察到显著交互作用,见图3。值得注意的是,对于≥60岁和无体力活动的骨关节炎"
| [1] JIANG Y. Osteoarthritis year in review 2021: biology. Osteoarthritis Cartilage. 2022;30(2):207-215. [2] ABRAMOFF B, CALDERA FE. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104(2):293-311. [3] VINA ER, KWOH CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160-167. [4] CONSTANTINO DE CAMPOS G, MUNDI R, WHITTINGTON C, et al. Osteoarthritis, mobility-related comorbidities and mortality: an overview of meta-analyses. Ther Adv Musculoskelet Dis. 2020;12: 1759720X20981219. [5] MENDY A, PARK J, VIEIRA ER. Osteoarthritis and risk of mortality in the USA: a population-based cohort study. Int J Epidemiol. 2018;47(6): 1821-1829. [6] KUANG X, CHIOU J, LO K, et al. Magnesium in joint health and osteoarthritis. Nutr Res. 2021;90:24-35. [7] VERONESE N, LA TEGOLA L, CARUSO MG, et al. The Association between Dietary Magnesium Intake and Magnetic Resonance Parameters for Knee Osteoarthritis. Nutrients. 2019;11(6):1387. [8] KONSTARI S, SARES-JÄSKE L, HELIÖVAARA M, et al. Dietary magnesium intake, serum high sensitivity C-reactive protein and the risk of incident knee osteoarthritis leading to hospitalization-A cohort study of 4,953 Finns. PloS one. 2019;14(3):e0214064. [9] WU Z, YANG J, LIU J, et al. The relationship between magnesium and osteoarthritis of knee: A MOOSE guided systematic review and meta-analysis. Medicine (Baltimore). 2019;98(45):e17774. [10] BAI R, MIAO MZ, LI H, et al. Increased Wnt/β-catenin signaling contributes to autophagy inhibition resulting from a dietary magnesium deficiency in injury-induced osteoarthritis. Arthritis Res Ther. 2022; 24(1):165. [11] SHI Z, ABOU-SAMRA AB. Association of low serum magnesium with diabetes and hypertension: Findings from Qatar Biobank study. Diabetes Res Clin Pract. 2019;158:107903. [12] KOSTOV K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int J Mol Sci. 2019;20(6):1351. [13] TANGVORAPHONKCHAI K, DAVENPORT A. Magnesium and Cardiovascular Disease. Adv Chronic Kidney Dis. 2018;25(3): 251-260. [14] FRITZEN R, DAVIES A, VEENHUIZEN M, et al. Magnesium Deficiency and Cardiometabolic Disease. Nutrients. 2023;15(10):2355. [15] WANG M, PENG J, YANG C, et al. Magnesium intake and all-cause mortality after stroke: a cohort study. Nutr J. 2023;22(1):54. [16] EVERS I, CRUIJSEN E, KORNAAT I, et al. Dietary magnesium and risk of cardiovascular and all-cause mortality after myocardial infarction: A prospective analysis in the Alpha Omega Cohort. Front Cardiovasc Med. 2022;9:936772. [17] MENDES PMV, BEZERRA DLC, DOS SANTOS LR, et al. Magnesium in Breast Cancer: What Is Its Influence on the Progression of This Disease? Biol Trace Elem Res. 2018;184(2):334-339. [18] DANA N, KARIMI R, MANSOUrian M, et al. Magnesium intake and lung cancer risk: A systematic review and meta-analysis. Int J Vitam Nutr Res. 2021;91(5-6):539-546. [19] BORRUD L, CHIAPPA MM, BURT VL, et al. National Health and Nutrition Examination Survey: national youth fitness survey plan, operations, and analysis, 2012. Vital Health Stat 2. 2014;(163):1-24. [20] AHLUWALIA N, DWYER J, TERRY A, et al. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv Nutr. 2016;7(1):121-34. [21] BRÄMER GR. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q. 1988; 41(1):32-36. [22] DENG X, TAN Y. A national cross-sectional analysis of selenium intake and risk of osteoarthritis: NHANES 2003-2016. Front Public Health. 2023;10:1047605. [23] XU F, EARP JE, ADAMI A, et al. The Relationship of Physical Activity and Dietary Quality and Diabetes Prevalence in US Adults: Findings from NHANES 2011-2018. Nutrients. 2022;14(16):3324. [24] LI Y, YUAN X, ZHENG Q, et al. The association of periodontal disease and oral health with hypertension, NHANES 2009-2018. BMC Public Health. 2023;23(1):1122. [25] MENDES MA, DA SILVA I, RAMIRES V, et al. Metabolic equivalent of task (METs) thresholds as an indicator of physical activity intensity. PloS One. 2018;13(7):e0200701. [26] BIJLSMA JW, BERENBAUM F, LAFEBER FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115-2126. [27] YUSUF E, NELISSEN RG, IOAN-FACSINAY A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69(4):761-765. [28] LOUATI K, VIDAL C, BERENBAUM F, et al. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015;1(1):e000077. [29] ALBERTI KG, ZIMMET P, SHAW J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059-1062. [30] ZHUO Q, YANG W, CHEN J, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729-737. [31] PUENPATOM RA, VICTOR TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121(6):9-20. [32] KLUZEK S, NEWTON JL, ARDEN NK. Is osteoarthritis a metabolic disorder? Br Med Bull. 2015;115(1):111-121. [33] SAMPATH SJP, VENKATESAN V, GHOSH S, et al. Obesity, Metabolic Syndrome, and Osteoarthritis-An Updated Review. Curr Obes Rep. 2023;12(3):308-331. [34] RAINBOW R, REN W, ZENG L. Inflammation and Joint Tissue Interactions in OA: Implications for Potential Therapeutic Approaches. Arthritis. 2012;2012:741582. [35] MAGUIRE ME, COWAN JA. Magnesium chemistry and biochemistry. Biometals. 2002;15(3):203-210. [36] NIELSEN FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res. 201818;11:25-34. [37] BARBAGALLO M, BELVEDERE M, DOMINGUEZ LJ. Magnesium homeostasis and aging. Magnes Res. 2009;22(4):235-246. [38] CUBAŁA WJ, LANDOWSKI J, DZIADZIUSZKO M, et al. Magnesium, C-reactive protein, and cortisol in drug-naïve patients with short illness-duration, first episode major depressive disorder: possible immunomodulatory role for magnesium. Magnes Res. 2016;29(4):169-174. [39] WANG Y, WEI J, ZENG C, et al. Association between serum magnesium concentration and metabolic syndrome, diabetes, hypertension and hyperuricaemia in knee osteoarthritis: a cross-sectional study in Hunan Province, China. BMJ Open. 2018;8(9):e019159. [40] YAO H, XU JK, ZHENG NY, et al. Intra-articular injection of magnesium chloride attenuates osteoarthritis progression in rats. Osteoarthritis Cartilage. 2019;27(12):1811-1821. [41] VAN LAECKE S. Hypomagnesemia and hypermagnesemia. Acta Clin Belg. 2019;74(1):41-47. [42] MAIER JA, CASTIGLIONI S, LOCATELLI L, et al. Magnesium and inflammation: Advances and perspectives. Semin Cell Dev Biol. 2021; 115:37-44. [43] GROENENDIJK I, VAN DELFT M, VERSLOOT P, et al. Impact of magnesium on bone health in older adults: A systematic review and meta-analysis. Bone. 2022;154:116233. [44] FAA G, SABA L, FANNI D, et al. Association between Hypomagnesemia, COVID-19, Respiratory Tract and Lung Disease. Open Respir Med J. 2021;15:43-45. [45] BAGHERI A, NAGHSHI S, SADEGHI O, et al. Total, Dietary, and Supplemental Magnesium Intakes and Risk of All-Cause, Cardiovascular, and Cancer Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv Nutr. 2021;12(4):1196-1210. [46] QU X, JIN F, HAO Y, et al. Nonlinear association between magnesium intake and the risk of colorectal cancer. Eur J Gastroenterol Hepatol. 2013;25(3):309-318. [47] LIU H, ZHANG K, XIONG L. Dietary magnesium intake and rheumatoid arthritis patients’ all-cause mortality: evidence from the NHANES database. J Health Popul Nutr. 2024;43(1):112. [48] LU X, WANG A, LIU K, et al. Associations of Dietary Magnesium Intake with All-Cause and Cause-Specific Mortality Among Individuals with Gout and Hyperuricemia. Biol Trace Elem Res. 2024. doi: 10.1007/s12011-024-04395-y. [49] Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press (US), 1997. [50] CHEUNGPASITPORN W, THONGPRAYOON C, BATHINI T, et al. Impact of admission serum magnesium levels on long-term mortality in hospitalized patients. Hosp Pract (1995). 2020;48(2):80-85. [51] HAIDER DG, LINDNER G, AHMAD SS, et al. Hypermagnesemia is a strong independent risk factor for mortality in critically ill patients: results from a cross-sectional study. Eur J Intern Med. 2015;26(7):504-507. [52] TAN L, XU Q, LI C, et al. High-Normal Serum Magnesium and Hypermagnesemia Are Associated With Increased 30-Day In-Hospital Mortality: A Retrospective Cohort Study. Front Cardiovasc Med. 2021;8:625133. [53] LI J, IMTIAZ MS, BEARD NA, et al. ß-Adrenergic stimulation increases RyR2 activity via intracellular Ca2+ and Mg2+ regulation. PLoS One. 2013;8(3):e58334. [54] FIACCADORI E, DEL CANALE S, COFFRINI E, et al. Muscle and serum magnesium in pulmonary intensive care unit patients. Crit Care Med. 1988 Aug;16(8):751-60.Razzaque MS. Magnesium: Are We Consuming Enough? Nutrients. 2018;10(12):1863. [55] FELSENFELD AJ, LEVINE BS, RODRIGUEZ M. Pathophysiology of Calcium, Phosphorus, and Magnesium Dysregulation in Chronic Kidney Disease. Semin Dial. 2015;28(6):564-577. [56] YAMAGUCHI H, SHIMADA H, YOSHITA K, et al. Severe hypermagnesemia induced by magnesium oxide ingestion: a case series. CEN Case Rep. 2019;8(1):31-37. [57] RAZZAQUE MS. Magnesium: Are We Consuming Enough? Nutrients. 2018;10(12):1863. [58] LEIDI M, DELLERA F, MARIOTTI M, et al. High magnesium inhibits human osteoblast differentiation in vitro. Magnes Res. 2011;24(1):1-6. [59] CASTIGLIONI S, CAZZANIGA A, ALBISETTI W, et al. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5(8):3022-3033. [60] HAN H, FANG X, WEI X, et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: a systematic review and meta-analysis of prospective cohort studies. Nutr J. 2017;16(1):26. [61] FANG X, LIANG C, LI M, et al. Dose-response relationship between dietary magnesium intake and cardiovascular mortality: A systematic review and dose-based meta-regression analysis of prospective studies. J Trace Elem Med Biol. 2016;38:64-73. [62] WANG W, WANG X, CAO S, et al. Dietary Antioxidant Indices in Relation to All-Cause and Cause-Specific Mortality Among Adults With Diabetes: A Prospective Cohort Study. Front Nutr. 2022;9:849727. [63] KOLTE D, VIJAYARAGHAVAN K, KHERA S, et al. Role of magnesium in cardiovascular diseases. Cardiol Rev. 2014;22(4):182-192. [64] COURTIES A, GUALILLO O, BERENBAUM F, et al. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1955-1965. [65] KENDZERSKA T, JÜNI P, KING LK, et al. The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: a population-based cohort study. Osteoarthritis Cartilage. 2017;25(11):1771-1780. [66] SKOU ST, GRØNNE DT, ROOS EM. Prevalence, Severity, and Correlates of Pain Flares in Response to a Repeated Sit-to-Stand Activity: A Cross-sectional Study of 14 902 Patients With Knee and Hip Osteoarthritis in Primary Care. J Orthop Sports Phys Ther. 2020;50(6):309-318. [67] YUE M, ZHANG H, LI R. Meta-analysis on the risk of all-cause mortality and cardiovascular death in the early stage of hypertension. Pak J Pharm Sci. 2016;29(4 Suppl):1343-1351. [68] HUANG Y, SU L, CAI X, et al. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J. 2014; 167(2):160-168.e1. [69] BARBAGALLO M, VERONESE N, DOMINGUEZ LJ. Magnesium in Aging, Health and Diseases. Nutrients. 2021;13(2):463. [70] COUDRAY C, FEILLET-COUDRAY C, RAMBEAU M, et al. The effect of aging on intestinal absorption and status of calcium, magnesium, zinc, and copper in rats: a stable isotope study. J Trace Elem Med Biol. 2006;20(2):73-81. [71] NIELSEN FH, LUKASKI HC. Update on the relationship between magnesium and exercise. Magnes Res. 2006;19(3):180-189. |
| [1] | Chen Qiuhan, Yang Long, Yuan Daizhu, Wu Zhanyu, Zou Zihao, Ye Chuan. Peri-knee osteotomy for treatment of knee osteoarthritis: optimization of treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2303-2312. |
| [2] | Zhang Zizheng, Luo Wang, Liu Changlu. Application value of finite element analysis on unicompartmental knee arthroplasty for medial knee compartmental osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2313-2322. |
| [3] | Zhang Nan, Meng Qinghua, Bao Chunyu. Characteristics and clinical application of ankle joint finite element models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2343-2349. |
| [4] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [5] | Li Linzhen, Jiao Hongzhuo, Chen Weinan, Zhang Mingzhe, Wang Jianlong, Zhang Juntao. Effect of icariin-containing serum on lipopolysaccharide-induced inflammatory damage in human chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1368-1374. |
| [6] | Chen Ju, Zheng Jinchang, Liang Zhen, Huang Chengshuo, Lin Hao, Zeng Li. Effect and mechanism of beta-caryophyllene in mice with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1341-1347. |
| [7] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [8] | Li Hao, Tao Hongcheng, Zeng Ping, Liu Jinfu, Ding Qiang, Niu Chicheng, Huang Kai, Kang Hongyu. Mitogen-activated protein kinase signaling pathway regulates the development of osteoarthritis: guiding targeted therapy with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1476-1485. |
| [9] | Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224. |
| [10] | Zhang Qian, Huang Dongfeng. Weighted gene co-expression network analysis combined with machine learning to screen and validate biomarkers for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1096-1105. |
| [11] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [12] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [13] | Rong Xiangbin, , Zheng Haibo, Mo Xueshen, Hou Kun, Zeng Ping, . Plasma metabolites, immune cells, and hip osteoarthritis: causal inference based on GWAS data from European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1028-1035. |
| [14] | Gu Fucheng, Yang Meixin, Wu Weixin, Cai Weijun, Qin Yangyi, Sun Mingyi, Sun Jian, Geng Qiudong, Li Nan. Effects of Guilu Erxian Glue on gut microbiota in rats with knee osteoarthritis: machine learning and 16S rDNA analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1058-1072. |
| [15] | Li Xiaomin, Tian Xiangdong, Wang Chaolu. High tibial osteotomy on a single plane: femorofibular angle as a reference marker for mechanical axis correction [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 570-576. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||