Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (14): 3745-3752.doi: 10.12307/2026.614
Association between peri-implantitis and cytokine gene polymorphisms
Huang Rui, Wang Jie, Zhang Ye, Liao Jian
- College of Stomatology of Guizhou Medical University/Stomatological Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2025-04-12Accepted:2025-06-07Online:2026-05-18Published:2025-09-15 -
Contact:Liao Jian, PhD, Professor, Master’s supervisor, College of Stomatology of Guizhou Medical University/Stomatological Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Huang Rui, Master candidate, College of Stomatology of Guizhou Medical University/Stomatological Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:National Natural Science Foundation of China (Regional Project), No. 82260198 (to LJ)
CLC Number:
Cite this article
Huang Rui, Wang Jie, Zhang Ye, Liao Jian. Association between peri-implantitis and cytokine gene polymorphisms[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3745-3752.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
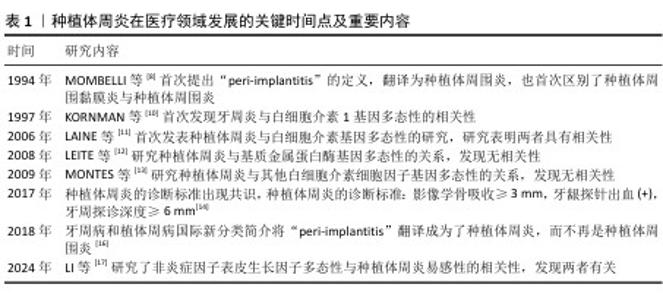
2.1 种植体周炎研究的发展 随着植入植体的数量增加,种植体周炎成为一个日益严重的问题。种植体周围炎在1994年被MOMBELLI等[8]提出,首次区分了种植体周围黏膜炎与种植体周围炎,并指出种植体周炎是伴随骨吸收的炎症性疾病。随着种植体周围炎的提出,针对其致病因素和发病机制的研究便如火如荼地进行中。研究者们认为种植体周围炎与牙周炎的临床表现比较相似,便从牙周炎的致病因素入手,试图寻找种植体周围炎的致病因素,发现种植体周围炎致病因素也包括吸烟、饮酒、代谢性疾病(如糖尿病)等与牙周炎相同的部分,另外,牙周炎也是种植体周围炎的危险因素[9]。然而,部分患者的牙周炎不能仅归因于外源性因素,可能还受遗传因素的影响。KORNMAN等[10]首次发现白细胞介素1的基因多态性与牙周炎严重程度相关,为后续种植体周围炎的遗传机制研究奠定基础。直到2006年,种植体周围炎与白细胞介素1基因多态性相关性的研究被首次发表,研究发现白细胞介素1基因多态性与种植体周围炎有关,白细胞介素1基因多态性可能代表该疾病的危险因素[11]。于是,打开了种植体周围炎与细胞因子基因多态性研究的大门,研究者们陆陆续续研究了基质金属蛋白酶、白细胞介素基因多态性和种植体周围炎的相关性[12-13],不同研究者得到了不同的结果,原因可能在于人群差异以及缺乏相同的诊断标准。针对种植体周围炎的诊断标准直到2017年才基本达成共识[14],遂在此之后针对种植周围炎有了一个共同的诊断标准。与此同时,在这年发表了以中国人群为研究对象关于种植体周围炎和肿瘤坏死因子基因多态性的研究[15],为之后分析人群中的遗传因素奠定了基础。而2018年根据牙周病和植体周病国际新分类简介,种植体周围炎更正为种植体周炎[16]。直到近期才逐渐出现研究非炎性细胞因子与种植体周炎相关性的研究[17]。种植体周炎发展的关键时间点见表1。"
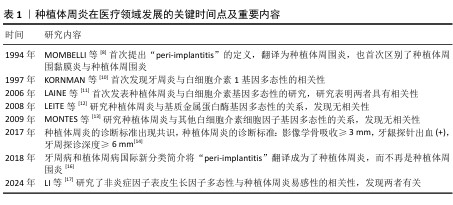

2.2 种植体周炎与炎症相关基因 2.2.1 种植体周炎与白细胞介素1基因多态性 白细胞介素1是一种多功能细胞因子,可促进牙槽骨吸收、细胞外基质破坏和破骨细胞生成,因此,在骨生理病理学中起着重要作用[18]。目前,在种植体周炎与白细胞介素基因多态性的研究中,白细胞介素1家族研究最为普遍与深入,但研究结论却存在差异。白细胞介素1家族由11个细胞因子成员组成,其中研究最多的3个成员是促炎细胞因子白细胞介素1α和白细胞介素1β,以及它们的拮抗蛋白白细胞介素1受体拮抗剂[4]。白细胞介素1α和白细胞介素1β能与相同受体结合,从而参与相关炎症过程。白细胞介素1受体拮抗剂通过结合与促炎性白细胞介素1相同的受体来阻止促炎信号的传递和免疫反应,从而发挥抗炎作用[19]。CARDOSO等[20]在102名受试者的样本中(种植体周炎患者∶健康种植体=43∶59)发现,白细胞介素1A-889、白细胞介素1B+3954、白细胞介素1RN(VNTR)基因多态性与种植体周炎发生并无关联。在较小的样本中也没有报告与白细胞介素1B+3954、白细胞介素1B-511和白细胞介素1RN+2018多态性与种植体周炎相关的证据[21-22]。然而,CARDOSO等[23]针对不同人群种植体周炎1A-889、种植体周炎1B+3954、种植体周炎1RN(VNTR)多态性与种植体周龈沟液中炎症递质浓度关系的研究,得到白细胞介素1受体拮抗剂的多态性可能会对种植体周炎发生产生影响的结果,这与MONTES等[13]的发现一致:白细胞介素1受体拮抗剂等位基因2的多态性与种植体周炎相关[13]。除此外,HAMDY等[24]指出,白细胞介素1等位基因2(种植体周炎1A-889和种植体周炎1B+3954组合)多态性患种植体周炎的风险更高。HE等[25]在超300受试者样本中也发现了种植体周炎1A-889、白细胞介素1B+3954多态性与种植体周炎相关。与此同时,一项纳入27项研究的Meta分析也同样显示,种植体周炎1A-889、种植体周炎1B+3954和-511可作为种植体周围疾病的预测标志物[26],该研究还关注到种植体周炎1A-889和种植体周炎1B+3954的复合基因型,与LAFUENTE-IBá?EZ等[27]和 MOHAMMADI等[28]的Meta分析结果一致,具有此复合基因型的个体患种植体周炎的风险更高。根据现有的研究仍不能完全明确白细胞介素1α和白细胞介素1β和白细胞介素1受体拮抗剂的多态性与种植体周炎的关系,原因可能在于所调查研究的样本量依然较小,高证据单核苷酸多态性研究通常需要更多的患者。 2.2.2 种植体周炎与白细胞介素4基因多态性 白细胞介素4基因多态性被称为“原型免疫调节细胞因子”,在调节抗体产生、造血和炎症及效应T细胞反应的发展中具有重要作用[29],其基因多态性与牙周炎的相关性已被广泛研究,但它与种植体周炎的关系尚未明确。白细胞介素4基因多态性与牙周炎的相关性研究广泛,但与种植体周炎的关系不明。PIGOSSI等[30]研究显示,白细胞介素4+33(C/T)多态性的C等位基因与种植体脱落相关,而白细胞介素4-590 C/T和白细胞介素4VNTR多态性的等位基因/基因型与种植体脱落无关。另外,LOPES等[31]研究发现正畸微小种植体脱落风险与白细胞介素4 rs2243268没有关系。 "


2.2.3 种植体周炎与白细胞介素6基因多态性 白细胞介素6是一种多效应细胞因子,可诱导B淋巴细胞分化、分泌免疫球蛋白、刺激破骨细胞前体的生长和分化、促进牙槽骨的吸收[32]。LISKMANN等[33]提出白细胞介素6可作为用于检测种植体周围组织疾病的有效标志物。高娟娟[34]在临床研究中发现,种植义齿修复患者白细胞介素6-174G/C基因多态性与种植体周围组织疾病的易感性具有相关性,认为该基因位点的多态性可能是增加种植体周炎风险的一个重要指标。CASADO等[35]也发现白细胞介素6-174-GG基因型和等位基因G的频率在健康组和患病组之间存在差异,可能是种植体周炎的危险因素。与之相反的是,PETKOVIC-CURCIN等[36]和MELO等[21]的临床研究以及李迎彩[37]和裴培等[38]的Meta分析都指出白细胞介素6-174G/C基因多态性与种植体周炎并无显著相关。 2.2.4 种植体周炎与白细胞介素10基因多态性 白细胞介素10是一种有效的抗炎细胞因子,可抑制几种促炎细胞因子的产生。白细胞介素10-1082 A/G(rs1800896)、白细胞介素10-819 C/T(rs1800871)和白细胞介素10-592 A/C(rs1800872)的多态性在白细胞介素10基因启动子区域可以检测到[39]。针对它们与种植体周炎的关系,SAREMI等[39]对50例种植体周炎患者的分析显示,白细胞介素10-819 C/T、白细胞介素10-592 C/A等位基因和基因型频率在该病患者和健康对照者之间存在显著差异,说明上述基因型可增加周肢体周炎的发病风险。但是,TAHA[40]在小样本实验中发现白细胞介素10-1082 A/G与种植体周炎发生无关,但观察到种植体周围疾病与白细胞介素10水平之间有显著关联,一项Meta分析也指出白细胞介素10-1082 A/G与种植体周炎发生无关联性[41]。 2.2.5 种植体周炎与白细胞介素17基因多态性 白细胞介素17是促炎症细胞因子,它可以结合受体的活化因子从而激活破骨细胞,造成骨吸收。杨文超等[42]分析健康种植体组和种植体周炎患者的样本发现,发白细胞介素17A-197G/A与口腔种植体周炎有相关性。同样,另一项研究也发现白细胞介素17-197G/A的基因多态性与种植体周炎具有相关性,其中GA、AA型可能是种植体周炎的危险基因型[43]。一项横断面研究也指出白细胞介素17A基因多态性可能在种植体周围疾病易感性中发挥作用[44]。以上研究都认为A等位基因可能是种植体周炎发病的危险因素。 2.2.6 种植体周炎与肿瘤坏死因子α 基因多态性 肿瘤坏死因子α是促炎细胞因子,可以诱导骨吸收。李芝香 等[45]在2023年的研究中提出,种植体周炎的发生和肿瘤坏死因子α-308多态性相关。ZHANG等[46]在一项纳入615例种植体周炎、795例健康种植体的Meta分析中也指出,肿瘤坏死因子α-308基因多态性可能会导致种植体周炎的易感。 另外,李丛丛等[15]也认为肿瘤坏死因子α-308位点可以成为种植体周炎的遗传学分子标记物。一项纳入98名受试者的临床研究和一项Meta分析发现,肿瘤坏死因子α-308基因多态性中的GA/AA基因型可能会增加种植体周炎的发病风险[36,41]。然而,一些研究认为肿瘤坏死因子α-308基因多态性与种植体周炎的发生并无直接关联[25-26,39]。 2.3 种植体周炎与免疫相关基因 基质金属蛋白酶是能够分解细胞外基质成分的酶,可能会导致牙周软硬组织的破坏[47]。对基质金属蛋白酶基因多态性与牙周炎易感关系的研究比较普遍,相关研究主要集中在基质金属蛋白酶1和基质金属蛋白酶13与种植体周炎的相关性上。一项针对60例未发生种植体失败患者和44例经历过种植体失败患者的研究表明,基质金属蛋白酶1-1607多态性中的GG基因型与早期种植体失败有关[12]。李迎彩[37]的研究也提基质金属蛋白酶1-1607的G等位基因与非吸烟人群的早期种植失败相关。但最近的一项病例报告发现,基质金属蛋白酶1-1607基因多态性与种植体周炎风险之间并无显著关联,还提出基质金属蛋白酶2-1306 C/T和基质金属蛋白酶13-77 A/G都与种植体周炎无关,基质金属蛋白酶3-1171基因多态性与种植体周炎发生风险有一定的相关 性[48]。 "


2.4 种植体周炎与骨代谢基因 骨保护素/核转录因子κB受体活化因子及其配体系统在控制骨吸收及骨形成的平衡中起着关键作用,其中,核转录因子κB受体活化因子配体与核转录因子κB受体活化因子相互作用诱导破骨细胞的形成和活化,导致骨吸收,骨保护素与核转录因子κB受体活化因子及其配体结合抑制骨吸收[49]。早期研究发现,核转录因子κB受体活化因子(rs35211496)CC基因型、核转录因子κB受体活化因子(rs9533156)TT基因型、骨保护素(rs2073617)CC基因型和骨保护素(rs2073618)CC基因型与种植体周炎相关[50]。近年来,姚舜等[51]在纳入90例种植体周围组织疾病患者和90名健康种植体的研究中发现,骨保护素-A163G基因位点的多态性可能与种植体周围组织疾病的发病相关。一项Meta分析也指出骨保护素rs2073618多态性极可能与种植体周炎的风险相关[52]。然而,RIBEIRO等[53]在巴西人群中未发现核转录因子κB受体活化因子相关基因多态性与种植体失败的关联。SILVA等[54]在针对核转录因子κB受体活化因子(rs3826620)、核转录因子κB受体活化因子(rs9594738)和骨保护素(rs2073618)3种基因多态性与种植体周炎关系的分析中,也未发现相关性。 "


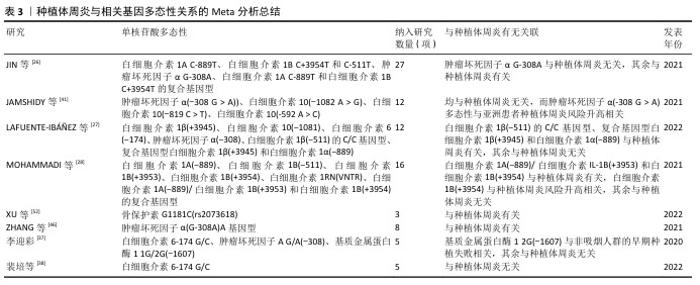
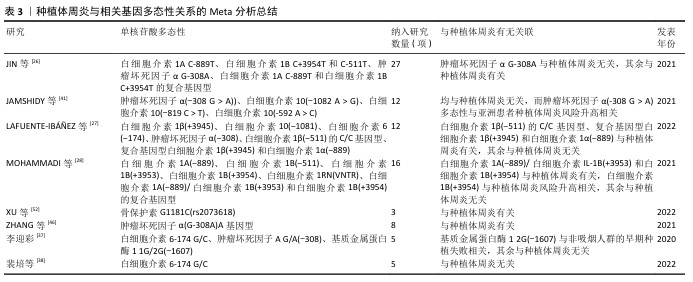
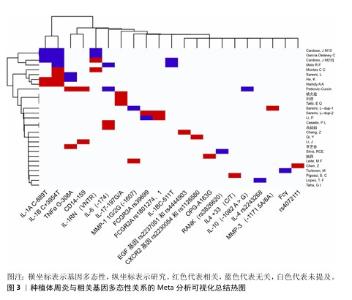
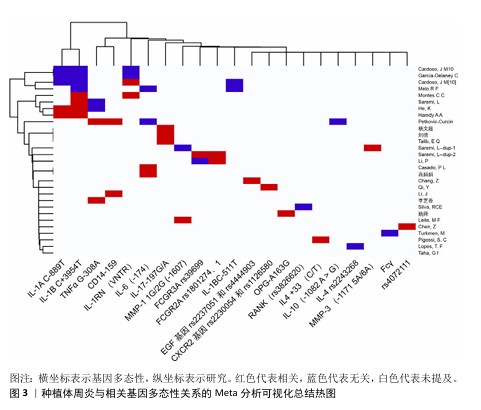
2.5 种植体周炎与其他因子基因 Fcγ受体是IgG的Fc区受体,可诱导单核细胞活化功能,如吞噬作用、脱颗粒、超氧化物生成、抗体依赖性细胞抑制、细胞因子产生和抗体调节,这些功能对宿主防御和免疫调节至关重要。Fcγ受体有3个亚家族,分别是 Fcγ受体Ⅰ、Ⅱ 和 Ⅲ[55]。既往研究已表明,Fcγ受体基因多态性会影响各种抗体介导的自身免疫和炎症性疾病的易感性[56]。SAREMI等[57]在对50例种植体周炎患者、87例慢性牙周炎患者和90例健康种植体基因多态性的分析中发现,FCGRIIa (rs1801274)、FCGRIIIa (rs396991)和FCGRIIIb (rs1050501)3个多态位点与慢性牙周炎和种植体周炎显著相关,验证了Fcγ受体的基因多态性对炎症性疾病易感性有所影响的结论。但是,LI等[17]针对FCGRIIIa (rs396991)位点的研究却得出了相反结论,认为FCGRIIIa (rs396991)与种植体周炎无关。CD14是结合内毒素脂多糖的多功能高亲和力受体,其可以呈比例地调节促炎细胞因子的产生,最终导致脂多糖刺激的骨吸收[58]。CD14多态性CD14-159 C/T被报道与种植体周炎相关,而CD14-1619 G/A与种植体周炎无关[59],该研究显示CD14-159 C/T中CC基因型和C等位基因是种植体周炎发生的危险因素,而另一项研究却认为CD14-159 C/T多态性CT/TT基因型与种植体周炎风险降低相关[36]。近年来,学者们开始关注一些另外因子多态性与疾病的关系,例如表皮生长因子以及趋化因子受体2。表皮生长因子是人体表皮细胞中的一种原始能量蛋白,可促进细胞增殖和分化、增强细胞代谢、促进表皮伤口愈合。表皮生长因子基因的异常表达在牙周炎患者中已被广泛报道[60]。CHANG等[61]在纳入300名受试者的研究中发现,表皮生长因子多态性与种植体周炎的发生相关。趋化因子受体2是趋化因子白细胞介素 8的特异性受体,它在口腔上皮细胞中表达。QI等[62]在纳入260名受试者的研究中发现,趋化因子受体2基因rs2230054和rs1126580多态性与中国汉族人群种植体周炎易感性相关。 该文通过系统性综述揭示了基因多态性在种植体周炎中的复杂作用,进一步说明了关于种植体周炎遗传因素的作用。根据现有的研究来看,基本可以明确遗传因素,特别是细胞因子基因多态性与种植体周炎的关系,也就是说,一些患种植体周炎并且口内呈聚集性的患者,他们的细胞因子基因多态性起着关键的作用。其中,已基本确定白细胞介素1β、白细胞介素6及基质金属蛋白酶基因多态性能与种植体周炎的发生有关。除此之外,该文既明确了部分新关联基因,也指出了现有争议的根源,为临床预防及治疗种植体周炎提供思路和参考。 种植体周炎与相关基因多态性关系的临床研究总结,见表2。种植体周炎与相关基因多态性关系的Meta分析总结,见表3。种植体周炎与相关基因多态性关系的Meta分析可视化总结热图,见图3。 "
| [1] SARTORETTO SC, SHIBLI JA, JAVID K, et al. Comparing the Long-Term Success Rates of Tooth Preservation and Dental Implants: A Critical Review. J Funct Biomater. 2023; 14(3):142. [2] COSGAREA R, SCULEAN A, SHIBLI JA, et al. Prevalence of peri-implant diseases - a critical review on the current evidence. Braz Oral Res. 2019;33(suppl 1):e063. [3] ROMANDINI M, LIMA C, PEDRINACI I, et al. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study. Clin Oral Implants Res. 2021;32(1):112-122. [4] FOURMOUSIS I, VLACHOS M. Genetic Risk Factors for the Development of Periimplantitis. Implant Dent. 2019;28(2): 103-114. [5] DARBY I. Risk factors for periodontitis & peri-implantitis. Periodontol 2000. 2022;90(1):9-12. [6] ALVIM-PEREIRA F, MONTES CC, MIRA MT, et al. Genetic susceptibility to dental implant failure: a critical review. Int J Oral Maxillofac Implants. 2008;23(3):409-416. [7] CHEN X, ZHAO Y. Genetic Involvement in Dental Implant Failure: Association With Polymorphisms of Genes Modulating Inflammatory Responses and Bone Metabolism. J Oral Implantol. 2019;45(4): 318-326. [8] MOMBELLI A, LANG NP. Microbial aspects of implant dentistry. Periodontol 2000. 1994;4:74-80. [9] CHMIELEWSKI M, PILLONI A. Current Molecular, Cellular and Genetic Aspects of Peri-Implantitis Disease: A Narrative Review. Dent J (Basel). 2023;11(5):134-134. [10] KORNMAN KS, CRANE A, WANG HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24(1):72-77. [11] LAINE ML, LEONHARDT A, ROOS-JANSÅKER AM, et al. IL-1RN gene polymorphism is associated with peri-implantitis. Clin Oral Implants Res. 2006;17(4):380-385. [12] LEITE MF, SANTOS MC, DE SOUZA AP, et al. Osseointegrated implant failure associated with MMP-1 promotor polymorphisms (-1607 and -519). Int J Oral Maxillofac Implants. 2008;23(4):653-658. [13] MONTES CC, ALVIM-PEREIRA F, DE CASTILHOS BB, et al. Analysis of the association of IL1B (C+3954T) and IL1RN (intron 2) polymorphisms with dental implant loss in a Brazilian population. Clin Oral Implants Res. 2009;20(2):208-217. [14] JEPSEN S, CATON JG, ALBANDAR JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45 Suppl 20:S219-S229. [15] 李丛丛,于玢玢,范春,等.TNF-α、IL-6分子基因多态性与种植体周围炎相关性分析[J].中国现代医药杂志,2017,19(2):1-5. [16] 郭淑娟,刘倩,丁一.牙周病和植体周病国际新分类简介[J].国际口腔医学杂志, 2019,46(2):125-134. [17] LI P, CHEN B, ZHAO L, et al. Correction: Correlations of FCGR2A 131R/H and FCGR3A 158V/F Polymorphisms with the Susceptibility of Peri-implantitis in Chinese Han Population. Mol Biotechnol. 2024;66(9):2677. [18] DINARELLO CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8-27. [19] LAINE ML, LEONHARDT A, ROOS-JANSÅKER AM, et al. IL-1RN gene polymorphism is associated with peri-implantitis. Clin Oral Implants Res. 2006;17(4):380-385. [20] CARDOSO JM, RIBEIRO AC, PROENÇA L, et al. Analysis of the Association of IL-1A, IL-1B, and IL-1RN Genetic Polymorphisms with Peri-implantitis in a Portuguese Population. Int J Oral Maxillofacm Implants. 2024;39(4):103-111. [21] MELO RF, LOPES BM, SHIBLI JA, et al. Interleukin-1β and interleukin-6 expression and gene polymorphisms in subjects with peri-implant disease. Clin Implant Dent Relat Res. 2012;14(6):905-914. [22] GARCÍA-DELANEY C, SÁNCHEZ-GARCÉS MÁ, FIGUEIREDO R, et al. Clinical significance of interleukin-1 genotype in smoking patients as a predictor of peri-implantitis: A case-control study. Med Oral Patol Oral Cir Bucal. 2015;20(6):e737-e743. [23] CARDOSO JM, RIBEIRO AC, BOTELHO J, et al. The Influence of Genetic Polymorphisms on the Expression of Interleukin-1beta, Prostaglandin E2 and Tumor Necrosis Factor Alpha in Peri-Implant Crevicular Fluid: A Cross-Sectional Study. Int J Mol Sci. 2024;25(1):651-651. [24] HAMDY AA, EBRAHEM MA. The effect of interleukin-1 allele 2 genotype (IL-1a(-889) and IL-1b(+3954)) on the individual’s susceptibility to peri-implantitis: case-control study. J Oral Implantol. 2011;37(3): 325-334. [25] HE K, JIAN F, HE T, et al. Analysis of the association of TNF-α, IL-1A, and IL-1B polymorphisms with peri-implantitis in a Chinese non-smoking population. Clin Oral Investig. 2020;24(2):693-699. [26] JIN Q, TENG F, CHENG Z. Association between common polymorphisms in IL-1 and TNFα and risk of peri-implant disease: A meta-analysis. PLoS One. 2021; 16(10):e0258138. [27] LAFUENTE-IBÁÑEZ DMI, SETIEN-OLARRA A, GARCÍA-DE LFA, et al. Role of proinflammatory mutations in peri-implantitis: systematic review and meta-analysis. Int J Implant Dent. 2022;8(1):2-2. [28] MOHAMMADI H, ROOCHI MM, SADEGHI M, et al. Association between Interleukin-1 Polymorphisms and Susceptibility to Dental Peri-Implant Disease: A Meta-Analysis. Pathogens. 2021;10(12):1600-1600. [29] BROWN MA, HURAL J. Functions of IL-4 and Control of Its Expression. Crit Rev Immunol. 2017;37(2-6):181-212. [30] PIGOSSI SC, ALVIM-PEREIRA F, ALVIM-PEREIRA CC, et al. Association of interleukin 4 gene polymorphisms with dental implant loss. Implant Dent. 2014;23(6):723-731. [31] LOPES TF, SOUZA CM, REICHOW AM, et al. Analysis of the association of IL4 polymorphisms with orthodontic mini-implant loss. Int J Oral Maxillofac Surg. 2019;48(7):982-988. [32] 张海英.白细胞介素-6及其受体基因多态性与中国西北人群慢性牙周炎的关联研究[D].兰州:兰州大学,2011. [33] LISKMANN S, VIHALEMM T, SALUM O, et al. Correlations between clinical parameters and interleukin-6 and interleukin-10 levels in saliva from totally edentulous patients with peri-implant disease. Int J Oral Maxillofac Implants. 2006;21(4):543-550. [34] 高娟娟.白介素6基因多态性与种植体周围组织疾病的相关性研究[D].沈阳: 中国医科大学,2020. [35] CASADO PL, VILLAS-BOAS R, DE MELLO W, et al. Peri-implant disease and chronic periodontitis: is interleukin-6 gene promoter polymorphism the common risk factor in a Brazilian population? Int J Oral Maxillofac Implants. 2013;28(1):35-43. [36] PETKOVIC-CURCIN A, ZELJIC K, CIKOTA-ALEKSIC B, et al. Association of Cytokine Gene Polymorphism with Peri-implantitis Risk. Int J Oral Maxillofac Implants. 2017; 32(5):e241-e248. [37] 李迎彩.IL-6 G/C(-174)、TNFA G/A(-308)和MMP-1 1G/2G(-1607)基因多态性与种植体周围炎易感性的meta分析[D].长春:吉林大学,2020. [38] 裴培,姬晓伟,韩祥祯.IL-6-174 G/C基因多态性与种植体周围病易感性的Meta分析[J].新疆医学,2022,52(11):1271-1275. [39] SAREMI L, SHAFIZADEH M, ESMAEILZADEH E, et al. Assessment of IL-10, IL-1ß and TNF-α gene polymorphisms in patients with peri-implantitis and healthy controls. Mol Biol Rep. 2021;48(3):2285-2290. [40] TAHA GI. Involvement of IL-10 gene polymorphism (rs1800896) and IL-10 level in the development of periimplantitis. Minerva Dent Oral Sci. 2024;73(5):264-271. [41] JAMSHIDY L, TADAKAMADLA SK, CHOUBSAZ P, et al. Association of IL-10 and TNF-α Polymorphisms with Dental Peri-Implant Disease Risk: A Meta-Analysis, Meta-Regression, and Trial Sequential Analysis. Int J Environ Res Public Health. 2021;18(14):7697-7697. [42] 杨文超,吴静静.口腔种植体周围炎与IL-17A、IL-17A-197G/A基因多态性、TNF-α的相关性分析[J].中国卫生检验杂志, 2023,33(14):1754-1757. [43] 刘倩.白细胞介素17基因多态性与种植体周围炎相关性研究[D].沈阳:中国医科大学,2021. [44] TALIB EQ, Taha GI. Involvement of interlukin-17A (IL-17A) gene polymorphism and interlukin-23 (IL-23) level in the development of peri-implantitis. BDJ Open. 2024;10(1):12. [45] 李芝香,黄英.TNF-α水平及基因多态性与种植体周围炎相关性分析[J].中国医药导刊,2023,25(1):67-70. [46] ZHANG X, ZHU X, SUN W. Association Between Tumor Necrosis Factor-α (G-308A) Polymorphism and Chronic Periodontitis, Aggressive Periodontitis, and Peri-implantitis: A Meta-analysis. J Evid Based Dent Pract. 2021;21(3):101528. [47] CHECCHI V, MARAVIC T, BELLINI P, et al. The Role of Matrix Metalloproteinases in Periodontal Disease. Int J Environ Res Public Health. 2020;17(14):4923-4923. [48] SAREMI L, SHAHBAZI S, GHAFFARI ME, et al. The Association of Matrix Metalloproteinase-1,-2,-3,-7and-13Gene Polymorphisms with Peri-Implantitis in an Iranian Population: A Case-Control Study. Clin Exp Dent Res. 2024;10(6):e70049. [49] YASUDA H, SHIMA N, NAKAGAWA N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95(7):3597-3602. [50] EGUIA DEL VALLE A, LÓPEZ-VICENTE J, MARTÍNEZ-CONDE R, et al. Current understanding of genetic polymorphisms as biomarkers for risk of biological complications in implantology. J Clin Exp Dent. 2018;10(10):e1029-e1039. [51] 姚舜,刘倩,邓巍.骨保护素(OPG)基因多态性与种植体周围组织疾病的相关性研究[J].实用口腔医学杂志,2021, 37(4):525-529. [52] XU M, ZHANG C, HAN Y, et al. Association between Osteoprotegerin rs2073618 polymorphism and peri-implantitis susceptibility: a meta-analysis. BMC Oral Health. 2022;22(1):598. [53] RIBEIRO R, MELO R, TORTAMANO NP, et al. Polymorphisms of Il-10 (-1082) and RANKL (-438) Genes and the Failure of Dental Implants. Int J Dent. 2017;2017:3901368. [54] SILVA R, REIS M, ARID J, et al. Association between Genetic Polymorphisms in RANK, RANKL and OPG and Peri-Implant Diseases in Patients from the Amazon Region. Braz Dent J. 2020;31(1):63-68. [55] VAN SORGE NM, VAN DER POL WL, VAN DE WINKEL JG. FcgammaR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61(3):189-202. [56] CASTRO-DOPICO T, CLATWORTHY MR. IgG and Fcγ Receptors in Intestinal Immunity and Inflammation. Front Immunol. 2019;10:805. [57] SAREMI L, ESMAEILZADEH E, GHORASHI T, et al. Association of Fc gamma-receptor genes polymorphisms with chronic periodontitis and Peri-Implantitis. J Cell Biochem. 2019;120(7):12010-12017. [58] CIESIELSKA A, MATYJEK M, KWIATKOWSKA K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78(4):1233-1261. [59] LI J, QIAO X, SHANG J. Association analysis between CD14 gene polymorphisms and peri-implantitis susceptibility in a Chinese population. Immun Inflamm Dis. 2024;12(4):e1230. [60] ANGIERO F, UGOLINI A, CATTONI F, et al. Evaluation of bradykinin, VEGF, and EGF biomarkers in gingival crevicular fluid and comparison of PhotoBioModulation with conventional techniques in periodontitis: a split-mouth randomized clinical trial. Lasers Med Sci. 2020;35(4):965-970. [61] CHANG Z, JIANG D, ZHANG S, et al. Genetic association of the epidermal growth factor gene polymorphisms with peri-implantitis risk in Chinese population. Bioengineered 2021;12(1):8468-8475. [62] QI Y, LI C, DU Y, et al. Chemokine Receptor 2 (CXCR2) Gene Polymorphisms and Their Association with the Risk of Developing Peri-Implantitis in Chinese Han Population. J Inflamm Res. 2021;14:1625-1631. [63] WANG J, ZHANG Y, CAO J, et al. The role of autophagy in bone metabolism and clinical significance. Autophagy. 2023;19(9): 2409-2427. [64] OZKOCER O, OZKOCER SE, GULER B, et al. Immunohistochemical analysis with apoptosis and autophagy markers in periodontitis and peri-implantitis: Clinical comparative study. J Periodontal Res. 2023; 58(2):456-464. [65] CHENG Y, JIN W, ZHENG L, et al. The role of autophagy in SIM mediated anti-inflammatory osteoclastogenesis through NLRP3 signaling pathway. Immun Inflamm Dis. 2024;12(1):e1145. |
| [1] | Yang Lixia, Diao Liqin, Li Hua, Feng Yachan, Liu Xin, Yu Yuexin, Dou Xixi, Gu Huifeng, Xu Lanju. Regulatory mechanism of recombinant type III humanized collagen protein improving photoaging skin in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1988-2000. |
| [2] | Chen Haojie, Wang Dai, Shen Shan. Immune inflammatory microenvironment mechanisms in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2054-2062. |
| [3] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| [4] | Zhu Bingbing, He Haibin, Deng Jianghua, Wang Wenqiang, Mu Xiaoling. Human umbilical cord mesenchymal stem cells overexpressing interleukin 8 receptor inhibit inflammation and promote vascular repair [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(1): 61-66. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||