Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (11): 2681-2690.doi: 10.12307/2026.112
Three-dimensional culture of stromal vascular fraction self-assembles into complex vascularized osteogenic organoids
Wu Jiazhou1, 2, Qian Tao2, Liu Zexian2, Wu Yanbin2, He Ying2, Li Yazhou2, Peng Jiang1, 2
- 1Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Institute of Orthopedics, Fourth Medical Center, Chinese PLA General Hospital, Beijing 100853, China
-
Received:2025-02-06Accepted:2025-06-09Online:2026-04-18Published:2025-09-02 -
Contact:Peng Jiang, MD, Professor, Chief physician, Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Institute of Orthopedics, Fourth Medical Center, Chinese PLA General Hospital, Beijing 100853, China -
About author:Wu Jiazhou, MS candidate, Department of Orthopedics, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China; Institute of Orthopedics, Fourth Medical Center, Chinese PLA General Hospital, Beijing 100853, China -
Supported by:National Key Research and Development Program of China, No. 2024YFA1108605 (to PJ)
CLC Number:
Cite this article
Wu Jiazhou, Qian Tao, Liu Zexian, Wu Yanbin, He Ying, Li Yazhou, Peng Jiang. Three-dimensional culture of stromal vascular fraction self-assembles into complex vascularized osteogenic organoids[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2681-2690.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
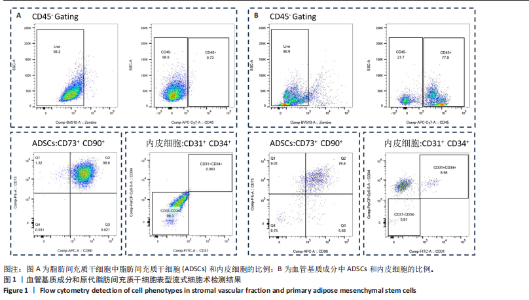
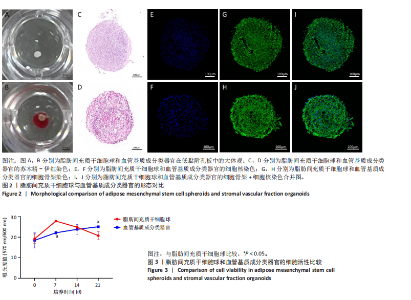
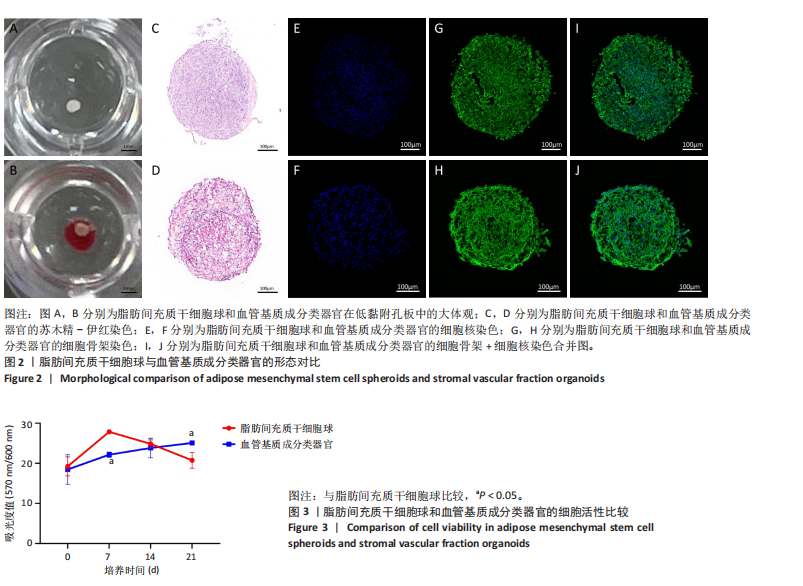
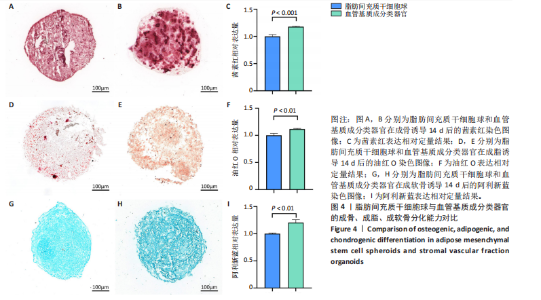
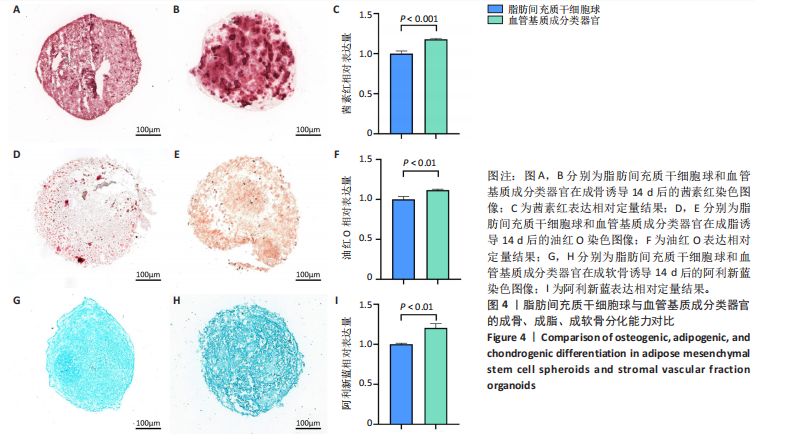
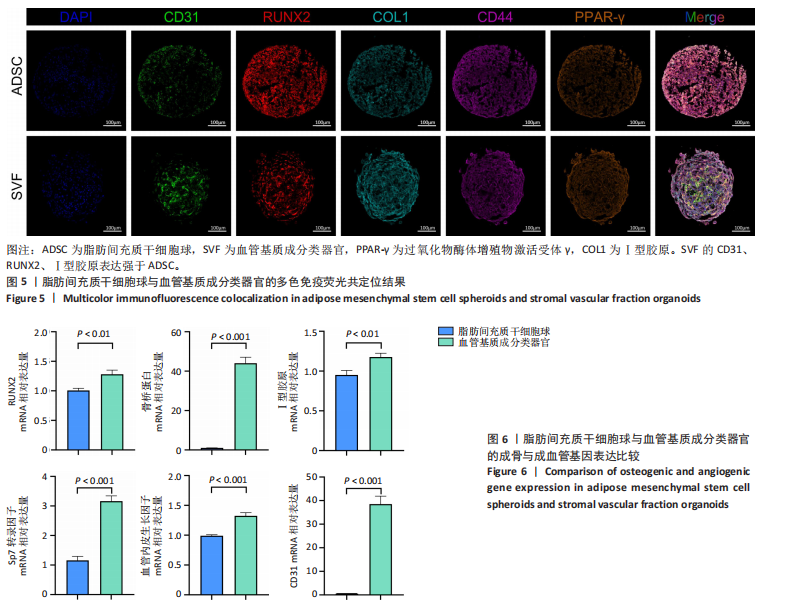
2.1 血管基质成分和原代脂肪间充质干细胞表型检测结果 流式细胞术检测结果显示,扩增培养后的原代脂肪间充质干细胞中的脂肪间充质干细胞(CD45-/CD73+/CD90+)比例较血管基质成分显著富集[(98.58±1.55)%,(70.9±9.17)%,P < 0.01],内皮祖细胞(CD45-/CD31+/CD34+)比例明显低于血管基质成分[(0.03±0.04)%,(6.04±2.95)%,P < 0.01],见图1。 2.2 血管基质成分类器官和脂肪间充质干细胞球大体观和细胞形态染色 图2A,B分别为脂肪间充质干细胞球和血管基质成分类器官在96孔超低黏附U型板中培养3 d后自组装聚集成球的宏观表现,可见两者尺寸无明显差异,但血管基质成分类器官底下有红细胞层衬托。苏木精-伊红、细胞骨架染色结果显示,脂肪间充质干细胞球表现为相同细胞的无序堆积,细胞外基质较少,细胞骨架无序且杂乱;血管基质成分类器官表现为更丰富的细胞外基质,细胞排列更加有序,细胞骨架也显示出更符合生理的结构特点,见图2C-J。 2.3 血管基质成分类器官和脂肪间充质干细胞球的细胞活性比较 成球培养第0,14天,血管基质成分类器官和脂肪间充质干细胞球的吸光度值比较差异无显著性意义(P > 0.05),脂肪间充质干细胞球成球培养第7天的吸光度值大于血管基质成分类器官 (P < 0.05)、成球培养第21天的吸光度值小于血管基质成分类器官(P < 0.05),见图3。在成球后21 d的培养过程中,脂肪间充质干细胞球的细胞活性先升高再下降,血管基质成分类器官的细胞活性始终保持上升趋势,说明血管基质成分类器官具有更持久更稳定的细胞活性。 2.4 血管基质成分类器官和脂肪间充质干细胞球成骨成脂成软骨分化能力 成骨诱导14 d后,血管基质成分类器官中矿化沉积的红染钙结节较脂肪间充质干细胞球更多、颗粒更大,茜素红相对平均表达量高于脂肪间充质干细胞球(P < 0.001),见图4A-C。成脂诱导14 d后,血管基质成分类器官和脂肪间充质干细胞球的红染脂质油滴都主要集中在球体外周,其中血管基质成分类器官的脂滴更大,血管基质成分类器官油红O相对平均表达量高于脂肪间充质干细胞球(P < 0.01),见图4D-F。成软骨诱导14 d后,血管基质成分类器官和脂肪间充质干细胞球阿利新蓝染色均可见阳性表达,其中血管基质成分类器官蓝染颜色更深,表明有更多的软骨特异性细胞外基质产生,血管基质成分类器官阿利新蓝相对平均表达量显著高于脂肪间充质干细胞球(P < 0.01),见图4G-I。上述结果证明两者均有三系诱导分化潜能,血管基质成分类器官的成骨成脂成软骨潜能明显强于脂肪间充质干细胞球。 2.5 血管基质成分类器官和脂肪间充质干细胞球多色免疫荧光共定位结果 成骨诱导14 d后免疫荧光染色共定位结果显示(图5),血管基质成分类器官中血管内皮细胞标志物CD31(绿色)表达明显,并且形成了血管网络状的结构,脂肪间充质干细胞球中CD31表达较弱;早期成骨标志物RUNX2(红色)在血管基质成分类器官中也形成了一种独特的分布,主要集中在球体内部并分布在内皮细胞周围,脂肪间充质干细胞球中的RUNX2呈现均匀分布;晚期成骨标志物Ⅰ型胶原(青色)在血管基质成分类器官中形成网络状的更符合生理特征的有序分布,而在脂肪间充质干细胞球中为颗粒状的散在分布且表达较弱;间充质干细胞标志物CD44(紫色)在两种球体中的表现相似,都较均匀地分布于整个球体;成脂标志物过氧化物酶体增殖物激活受体γ(橙色)在两种球体中的表达均较弱,说明成骨诱导分化后两种球体内的脂肪间充质干细胞成脂基因表达都在减弱,成脂能力降低。结果表明血管基质成分类器官形成了复杂且有序的内部结构,并且拥有良好的骨和血管生成能力。 2.6 血管基质成分类器官和脂肪间充质干细胞球体外成骨成血管基因表达检测 成骨诱导14 d后,qRT-PCR 检测结果显示,血管基质成分类器官成骨相关标志物RUNX2、骨桥蛋白、Ⅰ型胶原、Sp7转录因子mRNA 表达量均高于脂肪间充质干细胞球(P < 0.01或P < 0.001),成血管相关标志物CD31、血管内皮生长因子mRNA表达量均高于脂肪间充质干细胞球(P < 0.001),见图6。"

| [1] SIMIAN M, BISSELL MJ. Organoids: A historical perspective of thinking in three dimensions. J Cell Biol. 2017;216(1):31-40. [2] CHEN CS, MRKSICH M, HUANG S, et al. Geometric control of cell life and death. Science. 1997;276(5317):1425-1428. [3] SHAO Y, SANG J, FU J. On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology. Biomaterials. 2015;52:26-43. [4] VEGA SL, KWON MY, SONG KH, et al. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat Commun. 2018;9(1):614. [5] SASAI Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520-530. [6] WANG J, WU Y, LI G, et al. Engineering Large-Scale Self-Mineralizing Bone Organoids with Bone Matrix-Inspired Hydroxyapatite Hybrid Bioinks. Adv Mater. 2024;36(30):e2309875. [7] GROSSO A, BURGER MG, LUNGER A, et al. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front Bioeng Biotechnol. 2017;5:68. [8] LEE J, LEE S, AHMAD T, et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255:120192. [9] THERY A, BLÉRY P, MALARD O, et al. Role of the stromal vascular fraction from adipose tissue in association with a phosphocalcic scaffold in bone regeneration in an irradiated area. J Craniomaxillofac Surg. 2015; 43(7):1169-1176. [10] STAMNITZ S, KLIMCZAK A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells. 2021;10(8):1925. [11] ROHRINGER S, HOFBAUER P, SCHNEIDER KH, et al. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis. 2014; 17(4):921-933. [12] HOLNTHONER W, HOHENEGGER K, HUSA AM, et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med. 2015;9(2):127-136. [13] YAN Y, CHEN H, ZHANG H, et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials. 2019;190-191:97-110. [14] KWON HM, HUR SM, PARK KY, et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol. 2014;63(1):19-28. [15] WANG T, LI T, NIU X, et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol Direct. 2023;18(1):6. [16] HEPLER C, VISHVANATH L, GUPTA RK. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 2017;31(2):127-140. [17] HAN S, SUN HM, HWANG KC, et al. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit Rev Eukaryot Gene Expr. 2015;25(2):145-152. [18] FENNEMA EM, TCHANG LAH, YUAN H, et al. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med. 2018;12(1): e150-e158. [19] SINGH S, NYBERG EL, O’SULLIVAN AN, et al. Point-of-care treatment of geometrically complex midfacial critical-sized bone defects with 3D-Printed scaffolds and autologous stromal vascular fraction. Biomaterials. 2022;282:121392. [20] FILIPPI M, DASEN B, GUERRERO J, et al. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials. 2019;223:119468. [21] EPPLE C, HAUMER A, ISMAIL T, et al. Prefabrication of a large pedicled bone graft by engineering the germ for de novo vascularization and osteoinduction. Biomaterials. 2019;192:118-127. [22] WITTMANN K, DIETL S, LUDWIG N, et al. Engineering vascularized adipose tissue using the stromal-vascular fraction and fibrin hydrogels. Tissue Eng Part A. 2015;21(7-8):1343-1353. [23] VERMETTE M, TROTTIER V, MÉNARD V, et al. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials. 2007;28(18):2850-2860. [24] LOUIS F, SOWA Y, IRIE S, et al. Injectable Prevascularized Mature Adipose Tissues (iPAT) to Achieve Long-Term Survival in Soft Tissue Regeneration. Adv Healthc Mater. 2022;11(23):e2201440. [25] ESCUDERO M, VAYSSE L, EKE G, et al. Scalable Generation of Pre-Vascularized and Functional Human Beige Adipose Organoids. Adv Sci (Weinh). 2023;10(31):e2301499. [26] ROBLEDO F, GONZÁLEZ-HODAR L, TAPIA P, et al. Spheroids derived from the stromal vascular fraction of adipose tissue self-organize in complex adipose organoids and secrete leptin. Stem Cell Res Ther. 2023;14(1):70. [27] STROBEL HA, GERTON T, HOYING JB. Vascularized adipocyte organoid model using isolated human microvessel fragments. Biofabrication. 2021;13(3). doi: 10.1088/1758-5090/abe187. [28] LANCASTER MA, KNOBLICH JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014; 345(6194):1247125. [29] BROUTIER L, ANDERSSON-ROLF A, HINDLEY CJ, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11(9):1724-1743. [30] SATO T, VRIES RG, SNIPPERT HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009; 459(7244):262-265. [31] MILLS RJ, PARKER BL, QUAIFE-RYAN GA, et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell. 2019;24(6):895-907.e6. [32] MUN SJ, RYU JS, LEE MO, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71(5): 970-985. [33] LANCASTER MA, RENNER M, MARTIN CA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013; 501(7467):373-379. [34] DUTTA D, HEO I, CLEVERS H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol Med. 2017;23(5):393-410. [35] RANGA A, GJOREVSKI N, LUTOLF MP. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014;69-70:19-28. [36] KALUTHANTRIGE DON F, HUCH M. Organoids, Where We Stand and Where We Go. Trends Mol Med. 2021;27(5):416-418. [37] YAGHOUBI Y, MOVASSAGHPOUR A, ZAMANI M, et al. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [38] PAMPALONI F, REYNAUD EG, STELZER EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007; 8(10):839-845. [39] ZURINA IM, GORKUN AA, DZHUSSOEVA EV, et al. Human Melanocyte-Derived Spheroids: A Precise Test System for Drug Screening and a Multicellular Unit for Tissue Engineering. Front Bioeng Biotechnol. 2020;8:540. [40] LIU X, LI L, GAIHRE B, et al. Scaffold-Free Spheroids with Two-Dimensional Heteronano-Layers (2DHNL) Enabling Stem Cell and Osteogenic Factor Codelivery for Bone Repair. ACS Nano. 2022;16(2):2741-2755. [41] MULLER S, ADER I, CREFF J, et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep. 2019;9(1):7250. [42] ROATO I, BELISARIO DC, COMPAGNO M, et al. Adipose-Derived Stromal Vascular Fraction/Xenohybrid Bone Scaffold: An Alternative Source for Bone Regeneration. Stem Cells Int. 2018;2018:4126379. [43] CHAABAN M, MOYA A, GARCÍA-GARCÍA A, et al. Harnessing human adipose-derived stromal cell chondrogenesis in vitro for enhanced endochondral ossification. Biomaterials. 2023;303:122387. |
| [1] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [2] | Li Zhenyu, Zhang Siming, Bai Jiaxiang, Zhu Chen. Osthole improves osteogenic differentiation function of bone marrow mesenchymal stem cells under high-glucose conditions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1641-1648. |
| [3] | Jin Dongsheng, Zhao Zhanghong, Zhu Ziyin, Zhang Sen, Sun Zuyan, Deng Jiang. Effects of icariin-loaded microsphere-three-dimensional scaffold on osteogenic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1658-1668. |
| [4] | Zou Yulian, Chen Chaopei, Huang Haixia, Lan Yuyan, Liu Min, Huang Ting. Resveratrol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1669-1678. |
| [5] | Han Teng, Ma Hong, Yang Ruoyi, Luo Yi, Li Chao. Oral squamous cell carcinoma-derived exosomal delivery of angiopoietin-2 is involved in tumor angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1755-1767. |
| [6] | Wen Fan, Xiang Yang, Zhu Huan, Tuo Yanfang, Li Feng. Exercise improves microvascular function in patients with type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1225-1235. |
| [7] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| [8] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [9] | Zhou Zixiang, Zhao Baoxiang. Research progress in the relationship between nontraumatic necrosis of the femoral head and lipid metabolism and its treatment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 680-690. |
| [10] | Zuo Na, Tang Qi, Yu Meng, Tao Kai. Effect of miR-196b-5p in adipose-derived stem cell exosomes on burn wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 43-49. |
| [11] | Chen Qiheng, Weng Tujun, Peng Jiang. Effect of dimethylglyoxal glycine on osteogenic, adipogenesis differentiation, and mitophagy of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 50-57. |
| [12] | Liu Nian, Dong Xinyue, Wang Songpeng, Xu Yingjiang, Zhang Xiaoming. Stem cell exosomes and biomaterial-assisted exosomes in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 175-183. |
| [13] | Zhao Qianwei, Sun Guangyuan . Intestinal organoids: a bibliometric analysis of the latest trends in tissue/organ biology, disease modeling, and clinical applications [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 238-247. |
| [14] | Li Zikai, Zhang Chengcheng, Xiong Jiaying, Yang Xirui, Yang Jing, Shi Haishan. Potential effects of ornidazole on intracanal vascularization in endodontic regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-7. |
| [15] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||