Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (31): 6733-6742.doi: 10.12307/2025.536
Previous Articles Next Articles
Research on hair follicle organoids: current status, challenges and prospects
Wang Zhao, Gong Lin, Piao Yongjun
- Department of Dermatology, First Affiliated Hospital of Dalian Medical University, Dalian 116000, Liaoning Province, China
-
Received:2024-05-21Accepted:2024-08-05Online:2025-11-08Published:2025-02-25 -
Contact:Gong Lin, MD, Associate professor, Department of Dermatology, First Affiliated Hospital of Dalian Medical University, Dalian 116000, Liaoning Province, China -
About author:Wang Zhao, Master candidate, Department of Dermatology, First Affiliated Hospital of Dalian Medical University, Dalian 116000, Liaoning Province, China -
Supported by:National Natural Science Foundation Youth Scholars of China, No. 82003377 (to GL)
CLC Number:
Cite this article
Wang Zhao, Gong Lin, Piao Yongjun. Research on hair follicle organoids: current status, challenges and prospects[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6733-6742.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
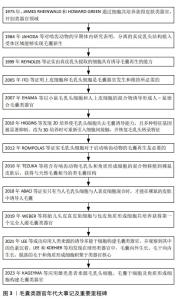
纵观毛囊类器官不断发展,诸多重要里程碑推动着毛囊再生的进展,如图3所示,最初毛乳头细胞可在体外诱导毛囊再生的潜力得到证实[9],随后研究发现上皮细胞与毛乳头细胞同时存在是毛囊发生的前提。在后续的发展中,学者们致力于探索不同来源的细胞共培养获得毛囊类器官的方法,直到2016年成功在啮齿动物移植模型中培养出类毛囊[10]。尽管在2007年时已有人-鼠嵌合毛囊类器官的研究[11],但第一个完全人毛囊类器官是WEBER等[12]于2019年构建的,随后,毛囊类器官研究逐渐转向难以解决的色素缺失及生产效率低下等难题。文章在简要描述毛囊形态发生及毛囊类器官形成原理的基础上,总结近年毛囊类器官的研究成果、培养方法及面临的挑战,以期能为后续研究提供思路。 "
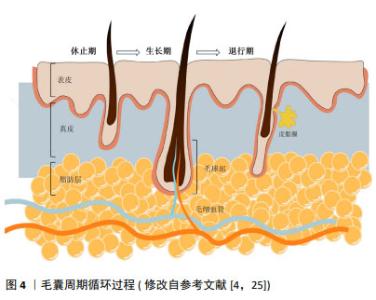
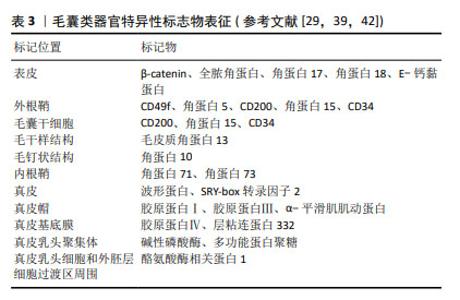
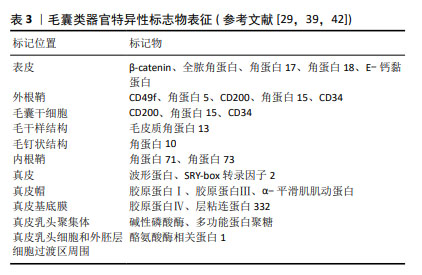
2.1 毛囊形态发生及周期调控 体内毛囊发育及毛发周期调控受到Wnt/β-catenin、音猬因子(sonic hedgehog pathway,SHH)通路、骨形态发生蛋白(bone morphogenetic protein pathway,BMP)通路、转化生长因子β(transforming growth factor-β,TGF-β)通路及Notch信号转导等多个通路调控[13]。在毛囊胚胎发育期,真皮中间充质细胞的Wnt/β-catenin信号激活是毛囊发育的始动因素,随后连续的上皮细胞增殖形成基板,SHH可通过促进角质形成细胞增殖间接促进基板向下形成毛胚[14]。真皮中的间充质细胞聚集在基板下方形成真皮凝聚物。此时周围BMP信号增加并抑制邻近表皮细胞的毛囊诱导[15],同时避免真皮凝聚物进一步增大。真皮凝聚物被上皮细胞包裹后形成真皮乳头,后者是毛囊中的间充质成分,其内包含毛乳头细胞、血管内皮细胞和细胞外基质[16-17]。这一过程中涉及上皮细胞与真皮乳头的相互作用,即上皮-间充质交互作用,在毛囊形态发生和周期循环中发挥重要调控作用。TGF-β通路主要在毛囊发育晚期通过与Wnt通路建立平衡以调控毛囊的下行生长[18]。随后进一步通过上皮-间充质交互作用促进毛钉和成熟毛囊上皮细胞增殖、分化,以在产前形成成熟毛囊[4],Notch信号通路可能通过调节上皮细胞间黏附参与这一过程[19]。 成熟毛囊以基底膜为界分为间充质室和上皮区室。间充质室中包括真皮乳头及真皮鞘,位于真皮鞘中的真皮鞘杯细胞是毛乳头细胞前体细胞,上皮区室由外向内依次为外根鞘、内根鞘、毛基质[20]。其中,位于外根鞘隆突的毛囊干细胞可分化为毛囊角质形成细胞,后者可在新一轮毛发周期开始前迁移至真皮乳头,经上皮-间充质交互作用分化成毛基质细胞[21-22]。隆突部位还存在黑素细胞,其产生的褐黑素和真黑素致使毛干色素沉着[5]。 成熟毛囊经历生长期、退行期和休止期循环[13]。Wnt信号蛋白主要在毛囊生长期表达,在退行期间减少,在休止期失活,β-连环蛋白是 Wnt 信号通路中的核心信号转导因子,通过激活淋巴样增强因子/T细胞因子复合物促进毛囊干细胞激活[23]。真皮巨噬细胞通过产生Wnt7b和Wnt10a激活毛囊干细胞启动生长期,后者在生长期分化形成内外根鞘及毛干[22]。BMP信号通路作用于休止期的不应期和再生期,在此期间BMP具有不同的信号活性强度[23],可维持毛囊干细胞静止,并促进毛囊角质形成细胞分化[24]。退行期基质内毛囊角质形成细胞、内根鞘细胞、外根鞘细胞及黑素细胞以不同方式死亡,真皮乳头通过调节高BMP和低Wnt信号沉默毛囊干细胞,使毛囊进入休止期[4],毛囊干细胞再次激活时可重新进入生长期,见图4[25]。 "

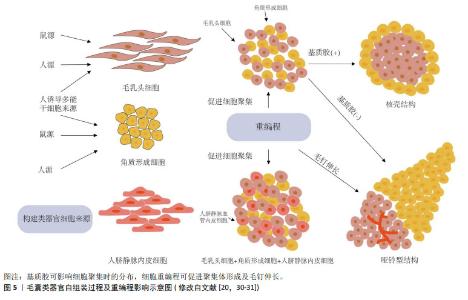
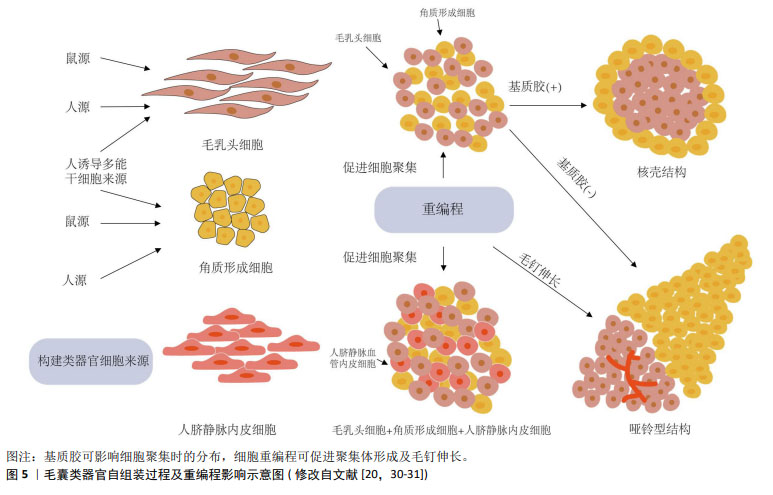
2.2 毛囊类器官形成的生物力学原理 类器官是由分离的组织祖细胞或多能干细胞经3D 培养产生,可形成与生理发育器官结构相似的微型器官[7]。毛乳头细胞在体外诱导毛囊形成的可行性早已被证实,通过复杂的级联信号途径影响邻近毛囊角质形成细胞,从而诱导非毛发表皮细胞形成毛囊[26],因此,毛乳头细胞是构建毛囊类器官理想的真皮细胞来源[20]。外根鞘隆突的毛囊角质形成细胞是毛囊所有上皮细胞来源[5],将其与毛乳头细胞共培养激发上皮-间充质交互作用是毛囊类器官形成的重要前提。 当不同类型细胞共培养时,基于差异黏附假说及其衍生的蒙特卡罗模拟实验表明,异质细胞间存在黏附性差异,当异质细胞类型之间的黏附性与均质细胞类型之间的黏附性较弱时,两种类型的细胞相互分离并最终形成哑铃形聚集体。相反,当异质细胞类型之间的黏附性与均质细胞类型之间的黏附性相当时,细胞形成由核心黏附细胞较多和外层黏附细胞较少组成的聚集体[27-28]。因此,当毛乳头细胞与毛囊角质形成细胞共培养时,初始构型主要由细胞间差异黏附性决定[29]。KAGEYAMA等[30]的研究证明了这一点,他们认为基质胶通过增加异质细胞间黏附性使其形成核壳结构,并增强上皮-间充质交互作用,而缺乏基质胶时,两种细胞黏附性减弱,向两端分离形成哑铃型,如图5所示[29-31]。这从生物力学角度解释了构建毛囊类器官时基质胶作为细胞外基质替代物的重要性,使调控细胞间黏附差异促进解离细胞构建毛囊类器官成为可能。 "

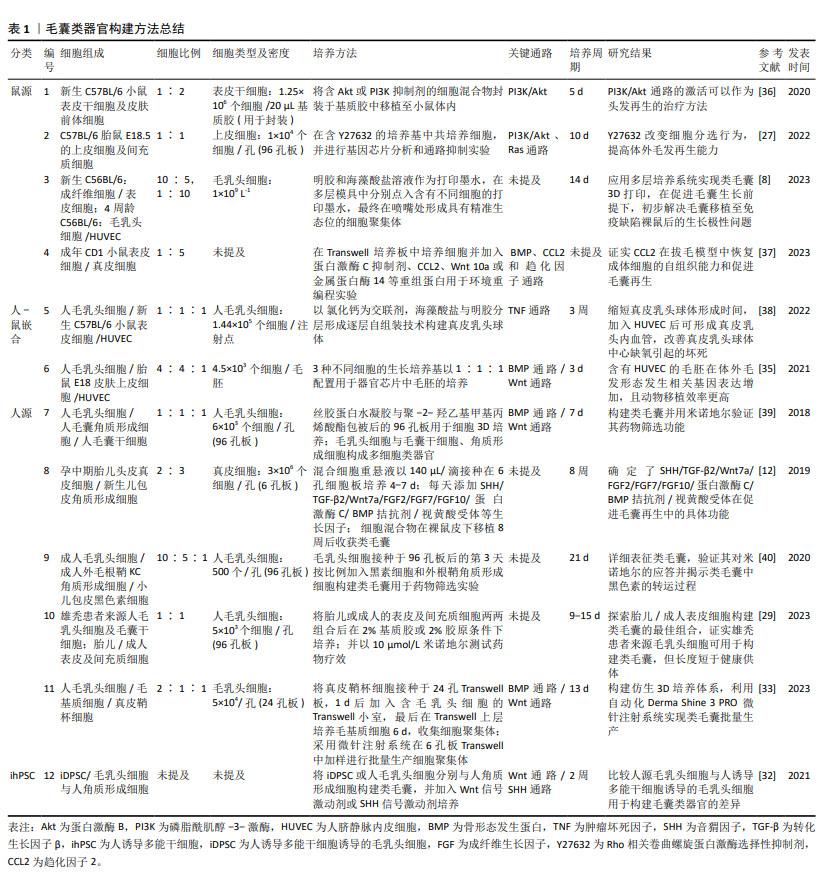
2.3 毛囊类器官培养的影响因素 再生医学研究进展已明确多种可能调节器官再生的因素,包括细胞外基质替代物组成、生物力学等[24]。毛囊类器官培养模仿实际的毛囊发育过程,因而毛乳头细胞来源及微环境对毛囊类器官培养至关重要;其次,还需考虑3D培养方式、培养条件改变对其形成和成熟的影响。 2.3.1 毛乳头细胞来源 目前毛囊类器官可以用小鼠或人源毛乳头细胞成功构建,其中包括人-鼠嵌合及完全人源毛囊类器官。实验性毛囊再生主要将角质形成细胞和毛乳头细胞共同移植到免疫缺陷小鼠体内[32],通过啮齿动物移植模型验证了体外解离细胞构建类毛囊的可能性[5]。 由于人和小鼠细胞在表皮-真皮多细胞构型的形态空间中存在不同发生途径[12],人-鼠嵌合毛囊类器官并不能直接作为移植材料治疗临床毛发疾病[33],因此,以解决临床问题为目的的毛囊类器官需以纯人源细胞作为起始原料。另外,人诱导多能干细胞是多种类器官的细胞来源,如软骨[34]、心脏等[3],研究表明人诱导多能干细胞也可分化为角质形成细胞前体细胞或真皮乳头替代细胞,并且FUKUYAMA等[32]以探索人源真皮乳头替代物为目的,通过诱导人诱导多能干细胞获得毛乳头细胞成功构建毛囊类器官,还发现与人源毛乳头细胞及角质形成细胞构建的类毛囊相比,人诱导多能干细胞来源毛乳头细胞及角质形成细胞构建的类毛囊中角蛋白33A、角蛋白82和淋巴样增强因子表达增强,尽管不能证实人诱导多能干细胞来源的毛乳头细胞及角质形成细胞更具优势,且仅有部分表型人诱导多能干细胞诱导的毛乳头细胞可形成真皮乳头聚集体[32],但至少有可能解决完全人源毛囊类器官材料短缺的问题。 如表1中总结[8,12,27,29,32-33,35-40],毛囊类器官构建方法涉及多种来源细胞的不同组合,根据细胞来源不同,将其分为鼠源、人-鼠嵌合、人源毛囊类器官以及人诱导多能干细胞构建的毛囊类器官。表中详细列出各类方法使用的细胞组成及细胞比例。简要总结了毛囊类器官培养基的组成,基质胶、培养基及部分生长因子添加剂是较为基础而易获得的培养条件,更为复杂的器官芯片[35]、仿生系统则需要复杂的技术支持[33]。在毛发相关信号通路方面,Wnt信号通路、BMP信号通路及SHH信号通路等仍较为重要。值得一提的是,在以毛囊类器官为模型的研究中,部分通路激活剂如PI3K/Akt通路激活剂[36]、SHH激动剂等被证实可促进毛发再生[32],可能对临床治疗脱发有一定的借鉴意义。再者,毛囊类器官的培养周期可能受多重因素影响,由解离细胞培养出毛胚结构需要的时间从几天至数周不等,提高毛囊类器官培养效率对其未来应用前景极为重要。由表中总结的研究成果不难看出,逐步趋于成熟的毛囊类器官培养方法使得患者自体细胞构建类毛囊成为可能,而其在药物筛选及工程化毛囊的尝试也许可为自体细胞构建类毛囊治疗脱发寻找新的出路。 "
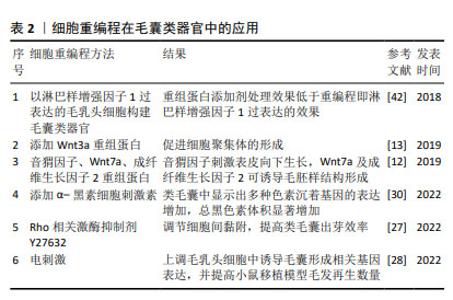
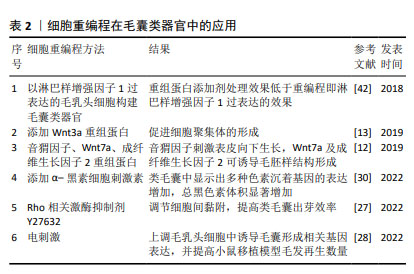
2.3.2 毛乳头细胞微环境 毛囊类器官的培养关键在于高度还原器官的体内微环境[41]。影响体内毛乳头细胞微环境的因素不仅包括细胞外基质,还包括各种邻近细胞,如毛基质细胞、血管内皮细胞和真皮鞘杯细胞、真皮巨噬细胞等的相互作用[33]。 (1)上皮-间充质交互作用:参与调节细胞间接触、细胞间基质相互作用[16],研究表明,将人源表皮细胞和毛乳头细胞共同移植至裸鼠体内,可实现毛囊形成[42],证实激活上皮-间充质交互作用是毛囊形成的重要前提[16]。对于这类双组分多细胞组装体,初始细胞比例及密度影响着形态空间中的最终稳定位置,这或许表明上皮-间充质交互作用的相对平衡和稳定有利于类毛囊的形成[12]。KAGEYAMA等[35]发现当细胞密度过高时,并不会形成更大的细胞聚集体,而是保持多个细胞聚集体,这可能与毛乳头细胞毛囊诱导特性保留有关。在细胞比例选择上,多数研究将相同数量的上皮细胞和毛乳头细胞共培养以得到毛囊类器官[27-28,31,43],少部分研究在同一条件下将来源相同的上皮细胞和毛乳头细胞按梯度比例共培养后,选择毛乳头细胞多于上皮细胞的培养模式[12,33,35]。如表1所示,在现有研究中,上皮细胞和毛乳头细胞来源不尽相同,培养条件各异,因此,难以确定两种细胞共培养的最佳比例和密度,KAGEYAMA等[35]和CHEN等[38]的梯度比例方法可提高科学探究的严谨性。 (2)细胞外基质:真皮乳头可分泌特定的细胞外基质,具有重要生理功能,其一是参与上皮-间充质交互作用调控,如成纤维细胞合成的多功能蛋白聚糖是真皮乳头的细胞外基质成分之一,可调节生长期毛囊Wnt信号转导[16];其次则是细胞外基质中的大分子蛋白利于毛囊微环境稳定,细胞外胶原蛋白可促进真皮乳头增殖和毛囊诱导特性的维持,而特异性蛋白聚糖(如聚集聚糖、大聚糖、纤连蛋白、透明质酸和Ⅰ型胶原)则具有促增殖作用[16]。因此,毛囊类器官依赖细胞外基质替代物进而生长、增殖及分化[44]。 (3)血管内皮细胞:真皮乳头中包括毛乳头细胞、血管内皮细胞及细胞外基质,由于毛囊类器官中缺乏脉管系统,因此3D培养的真皮乳头球体中心常因营养和氧气缺失发生坏死,CHEN等[38]在毛乳头细胞与表皮细胞共培养体系中加入人脐静脉内皮细胞,在类毛囊培养中可见血管形成,显著减少真皮乳头球体的坏死,并促进细胞增殖,同时完善了毛囊类器官中的脉管系统。另外,KAGEYAMA等[35]研究也证实了这一点,加入人脐静脉内皮细胞共培养不仅不影响类毛囊的形成过程,还使得毛胚在移植至小鼠体内后存活率增加,且产生更多数量新生毛发。因此,人脐静脉内皮细胞可能是毛囊类器官移植成活的关键因素之一,但同样需解决细胞密度及比例问题。 (4)钙黏蛋白:钙黏蛋白是典型的细胞间黏附分子,细胞的自发空间排列可能主要由细胞黏附分子的钙黏蛋白家族介导[28,45]。人毛乳头细胞、小鼠上皮细胞和小鼠胚胎间充质细胞具有不同的钙黏蛋白家族表达谱,它们分别表达钙黏蛋白11、钙黏蛋白2(N-钙黏蛋白)和钙黏蛋白1/3(E/P-钙黏蛋白)[28]。KAGEYAMA等[27]研究表明N-钙黏蛋白和E-钙黏蛋白均参与毛囊类器官形成过程,在加入Rho相关卷曲螺旋蛋白激酶选择性抑制剂Y-27632时,可下调间充质细胞中N-钙黏蛋白表达,从而增加上皮细胞和毛乳头细胞异质黏附率,促进毛囊类器官核壳聚集体形成。CHEN等[38]以钙离子作为交联剂,与明胶和海藻酸盐合成仿生细胞外基质,以此包被的毛乳头细胞在提高真皮乳头聚集效率的基础上,实现了真皮乳头球体大小的可控性。由此说明,钙黏蛋白过表达或使用人工修饰材料可作为调控细胞间黏附、提高毛囊类器官培养效率的方法之一。 (5)黑素细胞:黑素细胞是位于外根鞘隆突、与真皮乳头邻近的一类干细胞,可分为多个亚群[46],对新生毛发的色素沉着起调控作用。现有研究仍未能破译多种再生毛囊模型的色素缺失问题,即生出无色毛发,包括皮肤类器官[47]、毛囊类器官及创伤诱导的毛囊再生等。创伤诱导毛囊再生是当创伤面积足够大时在瘢痕中心发生的毛囊新生现象[48]。与创伤诱导毛囊再生不同的是,在毛囊类器官体系中加入黑素细胞时,可在黑素细胞中检测到多个阶段黑素体以及相邻毛囊角质形成细胞中运输的Ⅳ期囊泡[40]。观察皮肤或毛囊等类器官模型中黑素细胞的干细胞行为,有利于解决新生毛囊无色素难题。 (6)真皮巨噬细胞:真皮巨噬细胞密集分布于毛囊周围,主要是毛基质近端,参与毛乳头细胞免疫微环境的构成,并与毛发周期存在同步行为,即在毛囊退行期减少,生长期增加[49]。毛囊周围真皮巨噬细胞的异常分布与部分疾病如毛发扁平苔藓、压力性脱发密切相关。另外,WANG等[50]已明确创伤诱导毛囊再生模型巨噬细胞可通过TNF信号转导激活毛囊干细胞以促进毛囊再生。因此,真皮巨噬细胞在毛发再生中不可或缺,但其表型异质性与确切作用的关联性还需进一步阐明。CHU等[51]发现在机械拉伸诱导小鼠毛发再生模型中,皮下移植M2巨噬细胞比移植未极化或极化的M1巨噬细胞更能诱导毛发再生,而与之相悖的是WANG等[52]表明TREM2+真皮巨噬细胞某一亚群在休止期分泌抑癌蛋白M抑制毛发生长。将真皮巨噬细胞纳入毛囊类器官体系共培养,或许会进一步完善体外毛乳头细胞微环境,但前提是需明确真皮巨噬细胞不同表型、不同亚群对毛发再生的影响。 2.3.3 毛囊类器官的3D培养方式 毛乳头细胞保留着形成毛囊大小及其周期调控的记忆[1],体外2D培养的毛乳头细胞转录特征发生改变因而失去毛囊诱导特性,通过3D培养可部分恢复并维持这一特性,KANG等[8]研究还证明毛乳头细胞在3D培养下,其特异标志物表达显著高于贴壁培养。维持毛乳头细胞毛囊诱导特性是毛囊类器官培养的重要前提,也是近年来大量研究所努力的方向。因此,毛乳头细胞的3D培养方式选择和探索显得尤为重要。 目前现有的3D培养方式主要包括支架和无支架两种方法。支架可分为两类,一是根据不同类型的细胞或组织来制备具有不同大小孔径的人工或天然生物支架材料,二是微结构类似体内细胞外基质成分的合成或天然水凝胶,如胶原水凝胶[53],在类器官培养中充当细胞外基质,为细胞簇提供结构支持和细胞外基质信号[41]。基质胶作为一种常见支架,是Engelbreth-Holm-Swarm小鼠肉瘤细胞来源的异质凝胶状蛋白混合物,其主要成分是4种主要的基底膜细胞外基质蛋白:层粘连蛋白(约60%)、胶原Ⅳ(约30%)、巢蛋白(约8%)和硫酸肝素蛋白聚糖串珠素(2%-3%)[41,44]。由于基质胶属于肿瘤源性,其中残留的细胞外基质源性DNA、内毒素和炎性蛋白,可能干扰类器官生长和组织修复[54]。而且,在类器官临床研究和实际患者移植中,用小鼠基质胶培养类器官显然不太可能[33]。此外,天然和合成来源水凝胶,由于其成分明确和可调节的性质,是用于类器官培养的另一选择,但存在机械性能差、降解快、稳定性低等局限性[54]。合成水凝胶尽管在机械性能上有所提升,但存在生物相容性差、有毒降解产物等弊端[44]。 无支架技术,如球状体及细胞片等,前者包括悬滴培养及微流控装置。球状体技术在非支架依赖的类器官培养中,细胞聚集体形成效率比支架技术更高,同时避免支架技术使用细胞外基质替代物的多种不利因素[12,41]。然而,无支架技术缺乏细胞外基质替代物不利于某些细胞存活或增殖,未来或许可将相应组织的可溶性细胞外基质纳入无支架培养技术中,以同时获得兼具机械支撑及分化特异性的优点[55]。支架和无支架技术均可用于毛囊类器官培养,随着人们对其形成过程及培养条件的深入了解,结合毛囊类器官培养特点,或许可设计出适用于移植的可注射细胞外基质,以满足毛囊类器官用于治疗时的移植需求。 2.3.4 毛囊类器官的培养条件 3D培养,无论多么复杂,都缺乏结构完整性,如脉管系统、免疫系统和神经支配。目前尚无构建具有结构完整性的毛囊类器官的方法,但通过改变理化因素的环境重编程,在3D培养体系中加入毛发调控通路相关重组蛋白如Wnt3a[13]、Wnt7a、FGF2、SHH[12]、调节细胞间黏附的Rho相关卷曲螺旋蛋白激酶选择性抑制剂Y27632[27]、电刺激等[28],或遗传重编程,如构建毛乳头细胞中淋巴样增强因子1过表达模型[42],均能够提高真皮乳头聚集体形成效率或促进毛钉伸长,这对毛囊类器官的研究思路及培养方法极具参考价值,见表2。但需要注意的是,相关试剂的批次、供应商及实验室处理的差异,可能会影响最终实验结果的可重复性[6,56]。 "
| [1] LINDNER G, HORLAND R, WAGNER I, et al. De novo formation and ultra-structural characterization of a fiber-producing human hair follicle equivalent in vitro. J Biotechnol. 2011;152(3):108-112. [2] FOLTZ L, AVABHRATH N, LANCHY JM, et al. Craniofacial chondrogenesis in organoids from human stem cell-derived neural crest cells. iScience. 2024;27(4):109585. [3] LEE SW, SONG M, WOO DH, et al. Proposal for considerations during human iPSC-derived cardiac organoid generation for cardiotoxicity drug testing. Biomed Pharmacother. 2024;174:116511. [4] PARK S. Hair Follicle Morphogenesis During Embryogenesis, Neogenesis, and Organogenesis. Front Cell Dev Biol. 2022;10: 933370. [5] HOSSEINI M, KOEHLER KR, SHAFIEE A. Biofabrication of Human Skin with Its Appendage. Adv Healthc Mater. 2022;11(22):e2201626. [6] LEE J, VAN DER VALK WH, SERDY SA, et al. Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat Protoc. 2022;17(5):1266-1305. [7] WANG M, ZHOU X, ZHOU S, et al. Mechanical force drives the initial mesenchymal-epithelial interaction during skin organoid development. Theranostics. 2023;13(9):2930-2945. [8] KANG D, LIU Z, QIAN C, et al. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023; 165: 19-30. [9] REYNOLDS AJ, LAWRENCE C, CSERHALMI-FRIEDMAN PB, et al. Trans-gender induction of hair follicles. Nature. 1999;402(6757):33-34. [10] TEZUKA K, TOYOSHIMA KE, TSUJI T. Hair Follicle Regeneration by Transplantation of a Bioengineered Hair Follicle Germ. Methods Mol Biol. 2016;1453:71-84. [11] EHAMA R, ISHIMATSU-TSUJI Y, IRIYAMA S, et al. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. 2007;127(9):2106-2115. [12] WEBER EL, WOOLLEY TE, YEH CY, et al. Self-organizing hair peg-like structures from dissociated skin progenitor cells: New insights for human hair follicle organoid engineering and Turing patterning in an asymmetric morphogenetic field. Exp Dermatol. 2019;28(4):355-366. [13] SU Y, WEN J, ZHU J, et al. Pre-aggregation of scalp progenitor dermal and epidermal stem cells activates the WNT pathway and promotes hair follicle formation in in vitro and in vivo systems. Stem Cell Res Ther. 2019;10(1):403. [14] ABE Y, TANAKA N. Roles of the Hedgehog Signaling Pathway in Epidermal and Hair Follicle Development, Homeostasis, and Cancer. J Dev Biol. 2017;5(4):12. [15] SENNETT R, RENDL M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23(8): 917-927. [16] MAO MQ, JING J, MIAO YJ, et al. Epithelial-Mesenchymal Interaction in Hair Regeneration and Skin Wound Healing. Front Med (Lausanne). 2022;9:863786. [17] MARTEL JL, MIAO JH, BADRI T. Anatomy, Hair Follicle. Treasure Island (FL): StatPearls Publishing,2024. [18] TOMANN P, PAUS R, MILLAR SE, et al. Lhx2 is a direct NF-κB target gene that promotes primary hair follicle placode down-growth. Development. 2016;143(9):1512-1522. [19] WATT FM, ESTRACH S, AMBLER CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20(2): 171-179. [20] VATANASHEVANOPAKORN C, SARTYOUNGKUL T. iPSC-based approach for human hair follicle regeneration. Front Cell Dev Biol. 2023;11: 1149050. [21] FUCHS E. Scratching the surface of skin development. Nature. 2007; 445(7130):834-842. [22] WALL D, MEAH N, FAGAN N, et al. Advances in hair growth. Fac Rev. 2022;11:1. [23] LIN X, ZHU L, HE J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front Cell Dev Biol. 2022;10 899095. [24] YUE Z, LEI M, PAUS R, et al. The global regulatory logic of organ regeneration: circuitry lessons from skin and its appendages. Biol Rev Camb Philos Soc. 2021;96(6):2573-2583. [25] YANG M, WENG T, ZHANG W, et al. The Roles of Non-coding RNA in the Development and Regeneration of Hair Follicles: Current Status and Further Perspectives. Front Cell Dev Biol. 2021;9:720879. [26] HIGGINS CA, CHEN JC, CERISE JE, et al. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A. 2013;110(49):19679-19688. [27] KAGEYAMA T, ANAKAMA R, TOGASHI H, et al. Impacts of manipulating cell sorting on in vitro hair follicle regeneration. J Biosci Bioeng. 2022; 134(6):534-540. [28] YAN L, KAGEYAMA T, ZHANG B, et al. Electrical stimulation to human dermal papilla cells for hair regenerative medicine. J Biosci Bioeng. 2022;133(3):281-290. [29] KAGEYAMA T, MIYATA H, SEO J, et al. In vitro hair follicle growth model for drug testing. Sci Rep. 2023;13(1):4847. [30] KAGEYAMA T, SHIMIZU A, ANAKAMA R, et al. Reprogramming of three-dimensional microenvironments for in vitro hair follicle induction. Sci Adv. 2022;8(42):eadd4603. [31] KAGEYAMA T, SEO J, YAN L, et al. Cinnamic acid promotes elongation of hair peg-like sprouting in hair follicle organoids via oxytocin receptor activation. Sci Rep. 2024;14(1):4709. [32] FUKUYAMA M, TSUKASHIMA A, KIMISHIMA M, et al. Human iPS Cell-Derived Cell Aggregates Exhibited Dermal Papilla Cell Properties in in vitro Three-Dimensional Assemblage Mimicking Hair Follicle Structures. Front Cell Dev Biol. 2021;9:590333. [33] LIU Z, HUANG J, KANG D, et al. Microenvironmental reprogramming of human dermal papilla cells for hair follicle tissue engineering. Acta Biomater. 2023;165:31-49. [34] CHOI SH, LEE K, HAN H, et al. Prochondrogenic effect of decellularized extracellular matrix secreted from human induced pluripotent stem cell-derived chondrocytes. Acta Biomater. 2023;167:234-248. [35] KAGEYAMA T, CHUN Y S, FUKUDA J. Hair follicle germs containing vascular endothelial cells for hair regenerative medicine. Sci Rep. 2021;11:624. [36] CHEN Y, FAN Z, WANG X, et al. PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration. Stem Cell Res Ther. 2020;11(1): 144. [37] WU W, ZHOU W, JIANG J, et al. Mechanical stimuli-induced CCL2 restores adult mouse cells to regenerate hair follicles. Mol Ther Nucleic Acids. 2023;32:94-110. [38] CHEN P, MIAO Y, ZHANG F, et al. Tissue engineering ECM-enriched controllable vascularized human microtissue for hair regenerative medicine using a biomimetic developmental approach. J Adv Res. 2022;38:77-89. [39] GUPTA A C, CHAWLA S, HEGDE A, et al. Establishment of an in vitro organoid model of dermal papilla of human hair follicle. J Cell Physiol. 2018;233(11):9015-9030. [40] ATAÇ B, KISS FM, LAM T, et al. The microfollicle: a model of the human hair follicle for in vitro studies. In Vitro Cell Dev Biol Anim. 2020;56(10): 847-858. [41] CORRÒ C, NOVELLASDEMUNT L, LI V SW. A brief history of organoids. Am J Physiol Cell Physiol. 2020; 319(1): C151-C165. [42] ABACI HE, COFFMAN A, DOUCET Y, et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun. 2018;9(1):5301. [43] XIE S, CHEN L, ZHANG M, et al. Self-assembled complete hair follicle organoids by coculture of neonatal mouse epidermal cells and dermal cells in Matrigel. Ann Transl Med. 2022;10(14):767. [44] KAUR S, KAUR I, RAWAL P, et al. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58-66. [45] HAN W, HE M, ZHANG Y, et al. Cadherin-dependent adhesion modulated 3D cell-assembly. J Mater Chem B. 2022;10(26):4959-4966. [46] PALMER JW, KUMAR N, AN L, et al. Molecular heterogeneity of quiescent melanocyte stem cells revealed by single-cell RNA-sequencing. Pigment Cell Melanoma Res. 2024;37(4):480-495. [47] VAHAV I, VAN DEN BROEK LJ, THON M, et al. Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J Tissue Eng Regen Med. 2020;14(6):761-773. [48] GONG L, XIAO J, LI X, et al. IL-36α Promoted Wound Induced Hair Follicle Neogenesis via Hair Follicle Stem/Progenitor Cell Proliferation. Front Cell Dev Biol. 2020;8:627. [49] GAO Y, WANG J, ZHU DC, et al. Dermal macrophage and its potential in inducing hair follicle regeneration. Mol Immunol. 2021;134:25-33. [50] WANG X, CHEN H, TIAN R, et al. Macrophages induce AKT/β-catenin-dependent Lgr5+ stem cell activation and hair follicle regeneration through TNF. Nat Commun. 2017;8:14091. [51] CHU SY, CHOU CH, HUANG HD, et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat Commun. 2019;10(1):1524. [52] WANG ECE, DAI Z, FERRANTE AW, et al. A Subset of TREM2+ Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell Stem Cell. 2019;24(4): 654-669.e6. [53] 杜丽娟.基于器官原基组织工程原理构建单位毛囊微组织的实验研究[D].广州:南方医科大学,2023. [54] GAN Z, QIN X, LIU H, et al. Recent advances in defined hydrogels in organoid research. Bioact Mater. 2023;28:386-401. [55] VALDOZ JC, JOHNSON BC, JACOBS DJ, et al. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int J Mol Sci. 2021;22(23): 12690. [56] ATWOOD SX, PLIKUS MV. Fostering a healthy culture: Biological relevance of in vitro and ex vivo skin models. Exp Dermatol. 2021; 30(3):298-303. [57] SCHWEIZER J, LANGBEIN L, ROGERS MA, et al. Hair follicle-specific keratins and their diseases. Exp Cell Res. 2007;313(10):2010-2020. [58] KAO WWY. Keratin expression by corneal and limbal stem cells during development. Exp Eye Res. 2020;200:108206. [59] SILVA LMA, HSIEH R, LOURENÇO SV, et al. Immunostaining study of cytokeratins in human hair follicle development. An Bras Dermatol. 2020;95(3):278-282. [60] TOPOUZI H, LOGAN NJ, WILLIAMS G, et al. 2017Methods for the isolation and 3D culture of dermal papilla cells from human hair follicles. Exp Dermatol. 2017;26(6):491-496. [61] OHYAMA M. Use of human intra-tissue stem/progenitor cells and induced pluripotent stem cells for hair follicle regeneration. Inflamm Regen. 2019;39:4. [62] KRETZSCHMAR K, BOONEKAMP KE, BLEIJS M, et al. Troy/Tnfrsf19 marks epidermal cells that govern interfollicular epidermal renewal and cornification. Stem Cell Reports. 2021;16(9):2379-2394. [63] LEI M, LIN SJ, CHUONG CM. Editorial: Hair Follicle Stem Cell Regeneration in Aging. Front Cell Dev Biol. 2021;9:799268. [64] XIAO X, GAO Y, YAN L, et al. M1 polarization of macrophages promotes stress-induced hair loss via interleukin-18 and interleukin-1β. J Cell Physiol. 2024;239(4):e31181. [65] ALLMEROTH K, KIM CS, ANNIBAL A, et al. N1-acetylspermidine is a determinant of hair follicle stem cell fate. J Cell Sci. 2021;134(9): jcs252767. [66] KAGEYAMA T, YOSHIMURA C, MYASNIKOVA D, et al. Spontaneous hair follicle germ (HFG) formation in vitro, enabling the large-scale production of HFGs for regenerative medicine. Biomaterials. 2018;154: 291-300. [67] JEONG S, NA Y, NAM HM, et al. Skin-on-a-chip strategies for human hair follicle regeneration. Exp Dermatol. 2023;32(1):13-23. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [3] | Su Xiaoyang, Chen Wenting, Fu Yidan, Zhao Yan, Lan Danfeng, Yang Qiuping. Correlation between Mer receptor tyrosine kinase and diabetic peripheral neuropathy in Sprague-Dawley rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1593-1599. |
| [4] | Li Kaiying, Wei Xiaoge, Song Fei, Yang Nan, Zhao Zhenning, Wang Yan, Mu Jing, Ma Huisheng. Mechanism of Lijin manipulation regulating scar formation in skeletal muscle injury repair in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1600-1608. |
| [5] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [6] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [7] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [8] | Wang Xuanqiang, Zhang Wenyang, Li Yang, Kong Weiqian, Li Wei, Wang Le, Li Zhongshan, Bai Shi. Effects of chronic exposure to low-frequency pulsed magnetic fields on contractility and morphology of the quadriceps muscle in healthy adults [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1634-1642. |
| [9] | Zhang Yuxin, Yu Cong, Zhang Cui, Ding Jianjun, Chen Yan. Differences in postural control ability between older adults with mild cognitive impairment and those with normal cognition under different single-task and dual-task conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1643-1649. |
| [10] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [11] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [12] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [13] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [14] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [15] | Wang Yida, Liu Jun, Wang Xiaoling, Wang Liyan, Yang Chengru, Zhang Xuexiao. Effects of wearable electronic device-based interventions on physical activity and sedentary behavior in healthy adolescents: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1693-1704. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||