Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (25): 5403-5413.doi: 10.12307/2025.098
Previous Articles Next Articles
Coiled-coil-helix-coiled-coil-helix domain-containing 2 inhibits apoptosis of Parkinson’ s disease SH-SY5Y cells by promoting mitochondrial autophagy
Zhu Liuhui1, 2, Zhang Xinyue1, Zhu Zhouhai2, Yang Xinglong1, Guan Ying2, Liu Bin1, 2
- 1Department of Neurology, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China; 2Joint Institute of Smoking and Health, Kunming 650106, Yunnan Province, China
-
Received:2024-03-06Accepted:2024-04-28Online:2025-09-08Published:2024-12-26 -
Contact:Liu Bin, Master, Chief physician, Master’s supervisor, Department of Neurology, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China; Joint Institute of Smoking and Health, Kunming 650106, Yunnan Province, China; Co-corresponding author: Guan Ying, PhD, Associate researcher, Joint Institute of Smoking and Health, Kunming 650106, Yunnan Province, China -
About author:Zhu Liuhui, Master candidate, Department of Neurology, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China; Joint Institute of Smoking and Health, Kunming 650106, Yunnan Province, China -
Supported by:Applied Basic Research Foundation of Yunnan Province, No. 202101AY070001-115 (to LB); Open Fund of Joint Institute of Smoking and Health, No. 2021539200340039 (to YXL); Yunnan Provincial Education Department Scientific Research Fund Project, No. 2024Y226 (to ZLH)
CLC Number:
Cite this article
Zhu Liuhui, Zhang Xinyue, Zhu Zhouhai, Yang Xinglong, Guan Ying, Liu Bin. Coiled-coil-helix-coiled-coil-helix domain-containing 2 inhibits apoptosis of Parkinson’ s disease SH-SY5Y cells by promoting mitochondrial autophagy[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5403-5413.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
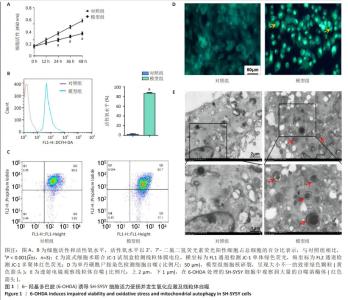
2.1 6-OHDA诱导SH-SY5Y细胞活力受损并发生氧化应激及线粒体自噬 6-OHDA是一种与多巴胺结构相似并能对多巴胺细胞产生毒性的氧化神经毒素,其处理的SH-SY5Y细胞被广泛用作帕金森病的体外模型。如图1A,B所示,与对照组相比,经6-OHDA处理的模型组细胞活性降低(P < 0.001),增殖速度降低,活性氧水平升高(P < 0.001)。如图1C所示,模型组细胞线粒体膜电位水平明显低于对照组[(0.83±0.05)% vs.(0.95±0.04)%,P < 0.01]。 由于过量的活性氧积累和低线粒体膜电位水平会破坏细胞内稳态并诱导自噬,使用单丹磺酰尸胺染色验证6-OHDA是否可以诱导细胞发生线粒体自噬。如图1D所示,对照组细胞呈现均匀的黄绿色染色,而模型组细胞核碎裂,呈现致密且大小不一的绿色染色颗粒[(0.87±0.03)% vs.(0.05±0.01)%,P < 0.001]。如图1E所示,透射电镜下模型组细胞较对照组细胞中存在大量自噬溶酶体[(8.65±2.67)/视野 vs. (0.20±0.05)/视野,P < 0.001],证实了线粒体自噬的存在。"

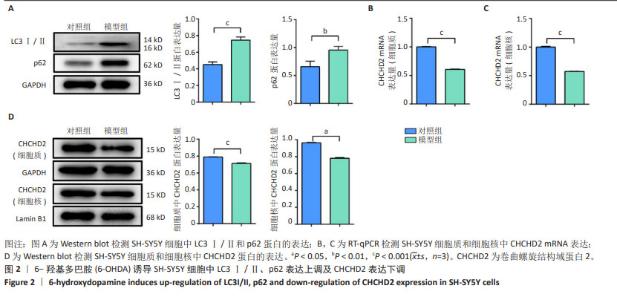
2.2 6-OHDA诱导SH-SY5Y细胞中LC3Ⅰ/Ⅱ、p62表达上调及CHCHD2表达下调 鉴于6-OHDA处理的SH-SY5Y细胞中观察到线粒体自噬现象,进一步分析了线粒体自噬标志物LC3Ⅰ/Ⅱ和p62的表达水平,如果表达水平升高则提示自噬水平降低[25]。如图2A所示,与对照组相比,模型组细胞中LC3Ⅰ/Ⅱ和p62表达水平显著上调(P < 0.001,P < 0.01)。CHCHD2定位于线粒体,在应激时转位至细胞核作为转录因子调控自身和其他蛋白如细胞色素氧化酶的表达[12],故通过mRNA和蛋白水平观察6-OHDA处理后的细胞质与细胞核中CHCHD2的表达变化。如图2B,C所示,与对照组相比,模型组细胞胞质和胞核中CHCHD2的mRNA表达明显下调(P < 0.001);如图2D所示,Western blot 检测结果也同样显示模型组细胞质和细胞核中CHCHD2蛋白表达较对照组均显著下调(P < 0.001,P < 0.05)。"

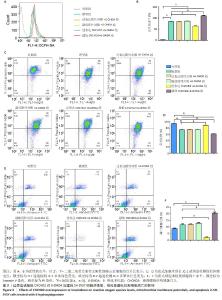
2.3 过表达或敲低CHCHD2对6-OHDA处理的SH-SY5Y细胞中活性氧、线粒体膜电位及细胞凋亡的影响 上述结果表明CHCHD2参与了自噬过程,为此在SH-SY5Y细胞中过表达CHCHD2或敲低CHCHD2的表达,进一步探究该蛋白在线粒体自噬中的作用。如图3A,B所示,过表达CHCHD2+6-OHDA组活性氧水平低于模型组(P < 0.001),而敲低CHCHD2+6-OHDA组活性氧水平高于模型组(P < 0.001)。如图3C,D所示,过表达CHCHD2+6-OHDA组线粒体膜电位水平显著高于模型组(P < 0.001),敲低CHCHD2+6-OHDA组线粒体膜电位水平低于模型组(P < 0.001)。此外,如图3E,F所示,CHCHD2过表达显著降低6-OHDA处理的SH-SY5Y细胞凋亡比例(P < 0.001),而CHCHD2敲低会促进相同条件下的细胞凋亡(P < 0.001)。"

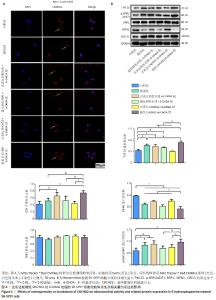
2.4 过表达或敲低CHCHD2对6-OHDA处理的SH-SY5Y细胞线粒体活性及相关蛋白表达的影响 线粒体凋亡途径是触发细胞凋亡的重要通路之一[26],使用Mito Tracker ? Red CMXRos探针观察过表达或敲低CHCHD2后6-OHDA处理的SH-SY5Y细胞线粒体的活性。如图4A所示,过表达CHCHD2 + 6-OHDA组[(89.25±3.33)%]和对照组[(86.65±2.50)%]的相对荧光强度高于模型组[(70.46±2.18)%],差异有显著性意义(P < 0.01),敲低CHCHD2+6-OHDA组[(64.67±2.55)%]的相对荧光强度低于模型组[(70.46±2.18)%],差异有显著性意义(P < 0.01)。过表达阴性对照+6-OHDA、敲低阴性对照+6-OHDA及模型组的相对荧光强度相近(P > 0.05)。如图4B所示,与对照组相比,模型组细胞中TIM23和p-DRP1的表达上调(P < 0.001,P < 0.05),MFN1的表达下调(P < 0.001);在6-OHDA处理的细胞中,过表达CHCHD2虽然对线粒体相关蛋白COXⅣ、TIM23和p-DRP1的表达没有明显影响(P > 0.05),但MFN1的表达显著上调(P < 0.001);而敲低CHCHD2后COXⅣ、TIM23和p-DRP1的表达显著上调(P < 0.01),MFN1的表达显著下调(P < 0.05)。"

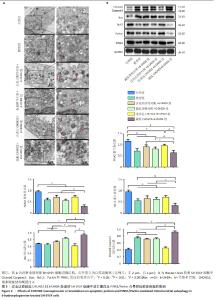
2.5 过表达或敲低CHCHD2对6-OHDA处理的SH-SY5Y细胞中凋亡蛋白及PINK1/Parkin介导的线粒体自噬的影响 如前所述,6-OHDA诱导SH-SY5Y细胞发生线粒体自噬,并在mRNA和蛋白水平抑制CHCHD2的表达。如图5A所示,透射电镜观察CHCHD2过表达促进线粒体自噬,可见大量自噬溶酶体(红色箭头),而敲低CHCHD2并用6-OHDA处理的SH-SY5Y细胞中自噬溶酶体的数量较模型组少。PINK1/Parkin通路是线粒体自噬的重要介导者,如图5B所示,模型组PINK1和Parkin的表达较对照组均有所下调,提示6-OHDA诱导的帕金森病细胞模型中此通路介导的线粒体自噬受损(P < 0.001);与模型组比较,过表达CHCHD2+6-OHDA组细胞中PINK1和Parkin表达显著上调(P < 0.01);而敲低CHCHD2+6-OHDA组细胞中PINK1和Parkin显著下调(P < 0.01)。此外,研究表明PINK1/Parkin介导的线粒体自噬可以抑制大鼠大脑皮质神经元凋亡而发挥重要的神经保护作用[26],因此检测了凋亡相关蛋白的表达以观察凋亡水平的变化,结果显示,Bcl-2的表达与PINK1和Parkin在相同条件下的表达趋势相似,而CHCHD2过表达时Bax和cleaved-caspase3的表达与模型组相比显著下调,在CHCHD2敲低时显著上调(P < 0.01)。"

| [1] YE H, ROBAK LA, YU M, et al. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu Rev Pathol. 2023;18:95-121. [2] BHIDAYASIRI R, KALIA LV, BLOEM BR. Tackling Parkinson’s Disease as a Global Challenge. J Parkinsons Dis. 2023;13(8):1277-1280. [3] KWON DK, KWATRA M, WANG J, et al. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells. 2022;11(23):3736. [4] MALPARTIDA AB, WILLIAMSON M, NARENDRA DP, et al. Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy. Trends Biochem Sci. 2021;46(4):329-343. [5] FUNAYAMA M, OHE K, AMO T, et al. CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol. 2015;14(3):274-282. [6] SHI CH, MAO CY, ZHANG SY, et al. CHCHD2 gene mutations in familial and sporadic Parkinson’s disease. Neurobiol Aging. 2016;38: 217.e9-217.e13. [7] YANG N, ZHAO Y, LIU Z, et al. Systematically analyzing rare variants of autosomal-dominant genes for sporadic Parkinson’s disease in a Chinese cohort. Neurobiol Aging. 2019;76:215.e1-215.e7. [8] JANSEN IE, BRAS JM, LESAGE S, et al. CHCHD2 and Parkinson’s disease. Lancet Neurol. 2015;14(7):678-679. [9] KOSCHMIDDER E, WEISSBACH A, BRÜGGEMANN N, et al. A nonsense mutation in CHCHD2 in a patient with Parkinson disease. Neurology. 2016;86(6):577-579. [10] IKEDA A, MATSUSHIMA T, DAIDA K, et al. A novel mutation of CHCHD2 p.R8H in a sporadic case of Parkinson’s disease. Parkinsonism Relat Disord. 2017;34:66-68. [11] LEE RG, SEDGHI M, SALARI M, et al. Early-onset Parkinson disease caused by a mutation in CHCHD2 and mitochondrial dysfunction. Neurol Genet. 2018;4(5):e276. [12] ZHOU W, MA D, TAN EK. Mitochondrial CHCHD2 and CHCHD10: Roles in Neurological Diseases and Therapeutic Implications. Neuroscientist. 2020;26(2):170-184. [13] ZHOU W, MA D, SUN AX, et al. PD-linked CHCHD2 mutations impair CHCHD10 and MICOS complex leading to mitochondria dysfunction. Hum Mol Genet. 2019;28(7):1100-1116. [14] ARAS S, BAI M, LEE I, et al. MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism. Mitochondrion. 2015;20:43-51. [15] LU Y, LI Z, ZHANG S, et al. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics. 2023;13(2):736-766. [16] MA KY, FOKKENS MR, REGGIORI F, et al. Parkinson’s disease-associated VPS35 mutant reduces mitochondrial membrane potential and impairs PINK1/Parkin-mediated mitophagy. Transl Neurodegener. 2021; 10(1):19. [17] MAGALHÃES JD, CARDOSO SM. Mitochondrial signaling on innate immunity activation in Parkinson disease. Curr Opin Neurobiol. 2023; 78:102664. [18] XU Y, ZHI F, MAO J, et al. δ-opioid receptor activation protects against Parkinson’s disease-related mitochondrial dysfunction by enhancing PINK1/Parkin-dependent mitophagy. Aging (Albany NY). 2020;12(24):25035-25059. [19] GLINKA Y, GASSEN M, YOUDIM MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997;50:55-66. [20] LIN CY, TSAI CW. PINK1/parkin-mediated mitophagy pathway is related to neuroprotection by carnosic acid in SH-SY5Y cells. Food Chem Toxicol. 2019;125:430-437. [21] DI RITA A, D’ACUNZO P, SIMULA L, et al. AMBRA1-Mediated Mitophagy Counteracts Oxidative Stress and Apoptosis Induced by Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells. Front Cell Neurosci. 2018;12:92. [22] WEI L, DING L, MO MS, et al. Wnt3a protects SH-SY5Y cells against 6-hydroxydopamine toxicity by restoration of mitochondria function. Transl Neurodegener. 2015;4:11. [23] YIN X, XIA J, SUN Y, et al. CHCHD2 is a potential prognostic factor for NSCLC and is associated with HIF-1a expression. BMC Pulm Med. 2020;20(1):40. [24] WEI JP, WEN W, DAI Y, et al. Drinking water temperature affects cognitive function and progression of Alzheimer’s disease in a mouse model. Acta Pharmacol Sin. 2021;42(1):45-54. [25] XIE L, GU Q, WU X, et al. Activation of LXRs Reduces Oxysterol Lipotoxicity in RPE Cells by Promoting Mitochondrial Function. Nutrients. 2022;14(12):2473. [26] WEN S, WANG L, ZHANG C, et al. PINK1/Parkin-mediated mitophagy modulates cadmium-induced apoptosis in rat cerebral cortical neurons. Ecotoxicol Environ Saf. 2022;244:114052. [27] KINET R, DEHAY B. Pathogenic Aspects and Therapeutic Avenues of Autophagy in Parkinson’s Disease. Cells. 2023;12(4):621. [28] SOOKHAKLARI R, GERAMIZADEH B, ABKAR M, et al. The neuroprotective effect of BSA-based nanocurcumin against 6-OHDA-induced cell death in SH-SY5Y cells. Avicenna J Phytomed. 2019;9(2):92-100. [29] ZHANG HY, JIANG YC, LI JR, et al. Neuroprotective effects of insulin-like growth factor-2 in 6-hydroxydopamine-induced cellular and mouse models of Parkinson’s disease. Neural Regen Res. 2023;18(5):1099-1106. [30] IKEDA A, IMAI Y, HATTORI N. Neurodegeneration-associated mitochondrial proteins, CHCHD2 and CHCHD10-what distinguishes the two? Front Cell Dev Biol. 2022;10:996061. [31] MENG H, YAMASHITA C, SHIBA-FUKUSHIMA K, et al. Loss of Parkinson’s disease-associated protein CHCHD2 affects mitochondrial crista structure and destabilizes cytochrome c. Nat Commun. 2017;8:15500. [32] LI J, YANG D, LI Z, et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res Rev. 2023;84:101817. [33] WANG S, LONG H, HOU L, et al. The mitophagy pathway and its implications in human diseases. Signal Transduct Target Ther. 2023; 8(1):304. [34] JIANG Y, KRANTZ S, QIN X, et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission - fusion dynamics and mitophagy. Redox Biol. 2022;52:102304. [35] JI W, REN Y, WEI X, et al. Ischemic stroke protected by ISO-1 inhibition of apoptosis via mitochondrial pathway. Sci Rep. 2023;13(1):2788. [36] AKABANE S, WATANABE K, KOSAKO H, et al. TIM23 facilitates PINK1 activation by safeguarding against OMA1-mediated degradation in damaged mitochondria. Cell Rep. 2023;42(5):112454. [37] YAPA NMB, LISNYAK V, RELJIC B, et al. Mitochondrial dynamics in health and disease. FEBS Lett. 2021;595(8):1184-1204. [38] JIANG T, WANG Y, WANG X, et al. CHCHD2 and CHCHD10: Future therapeutic targets in cognitive disorder and motor neuron disorder. Front Neurosci. 2022;16:988265. [39] ZHANG HT, MI L, WANG T, et al. PINK1/Parkin-mediated mitophagy play a protective role in manganese induced apoptosis in SH-SY5Y cells. Toxicol In Vitro. 2016;34:212-219. [40] GANGULY U, BANERJEE A, CHAKRABARTI SS, et al. Interaction of α-synuclein and Parkin in iron toxicity on SH-SY5Y cells: implications in the pathogenesis of Parkinson’s disease. Biochem J. 2020;477(6): 1109-1122. |
| [1] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [2] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [3] | Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898. |
| [4] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [5] | Su Qin, Jia Siwei, Guo Minfang, Meng Tao, Li Yanbing, Mu Bingtao, Song Lijuan, Ma Cungen, Yu Jiezhong. Lycium barbarum polysaccharide intervenes in SH-SY5Y cell injury induced by beta-amyloid protein 1-42: protective effect of mitochondrial autophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6688-6696. |
| [6] | Lin Shuqian, Zhao Xilong, Gao Jing, Pan Xinghua, Li Zian, Ruan Guangping. Comparison of biological characteristics of mouse bone marrow mesenchymal stem cells after interference and overexpression of telomere Cajal body protein-1 [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6616-6624. |
| [7] | Pan Yu, Zhao Renfeng, Li Xingping, Zhang Chengdong, Shi Feng, Pu Chao, Luo Xuwei, Xiao Dongqin. Iron overload induces ferroptosis in osteoblast precursor cells and inhibits osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6381-6390. |
| [8] | Zhang Xin, Guo Baojuan, Xu Huixin, Shen Yuzhen, Yang Xiaofan, Yang Xufang, Chen Pei. Protective effects and mechanisms of 3-N-butylphthalide in Parkinson’s disease cell models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6466-6473. |
| [9] | Nan Songhua, Peng Chaojie, Cui Yinglin. Mitochondrial dysfunction and brain aging: a bibliometrics analysis based on the Web of Science Core Collection database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5642-5651. |
| [10] | Jiang Qianping, Yang Dan, , , Wan Shilei, Xu Dandan, , , , Cao Lu, , Zhou Jing, , , . Role of O-linked N-acetylglucosamine glycosylation in neurodegenerative diseases and its clinical application prospects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5704-5712. |
| [11] | Lei Senlin, Chen Xiaoan, Chen Ping, Wang Zhaofeng. Exercise prevention and treatment of Parkinson’ s disease mediated by brain-derived neurotrophic factor: role and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5454-5468. |
| [12] | Guan Mengya, Ren Binbin, Wang Jingying. Major histocompatibility complex regulates immune responses in Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5469-5477. |
| [13] | Dong Guyu, Yu Jie, Zhao Lunan. Effect of Lycium barbarum polysaccharide on skeletal muscle mitochondria and antioxidant enzymes in rats undergoing heavy exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5134-5139. |
| [14] | Zhao Yuxin, Zhang Deqi, Bi Hongyan. Effect of different stimulation modalities of non-invasive brain stimulation on cognitive function in patients with Parkinson’s disease: a network Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5212-5223. |
| [15] | Tian Zhenli, Zhang Xiaoxu, Fang Xingyan, Xie Tingting. Effects of sodium arsenite on lipid metabolism in human hepatocytes and regulatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4956-4964. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||