Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (18): 3877-3884.doi: 10.12307/2025.657
Previous Articles Next Articles
The important role of the nervous system in regulating wound repair and scar healing
Song Jiamei1, Liu Tingting1, 2, Yao Bin1
- 1Academy of Medical Engineering and Translational Medicine, Tianjin University, Tianjin 300072, China; 2The 971th Hospital, Qingdao 266072, Shandong Province, China
-
Received:2024-06-21Accepted:2024-08-19Online:2025-06-28Published:2024-11-29 -
Contact:Yao Bin, PhD, Associate professor, Academy of Medical Engineering and Translational Medicine, Tianjin University, Tianjin 300072, China -
About author:Song Jiamei, Master candidate, Academy of Medical Engineering and Translational Medicine, Tianjin University, Tianjin 300072, China -
Supported by:the National Key Research and Development Program of China, Nos. 2022YFC2403100 and 2021YFF1200800 (to YB [project participant]); Natural Science Foundation of Tianjin, No. 21JCQNJC01070 (to YB); Applied Basic Research Foundation of Tianjin, Nos. 22JCQNJC00360 and 22JCQNJC00980 (to YB); Tianjin Health Research Project, No. TJWJ2023QN050 (to LTT); Shandong Province Natural Science Foundation Youth Project, No. ZR2021QC085 (to LTT)
CLC Number:
Cite this article
Song Jiamei, Liu Tingting, Yao Bin. The important role of the nervous system in regulating wound repair and scar healing[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3877-3884.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
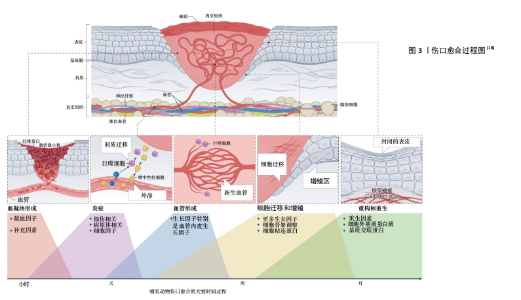
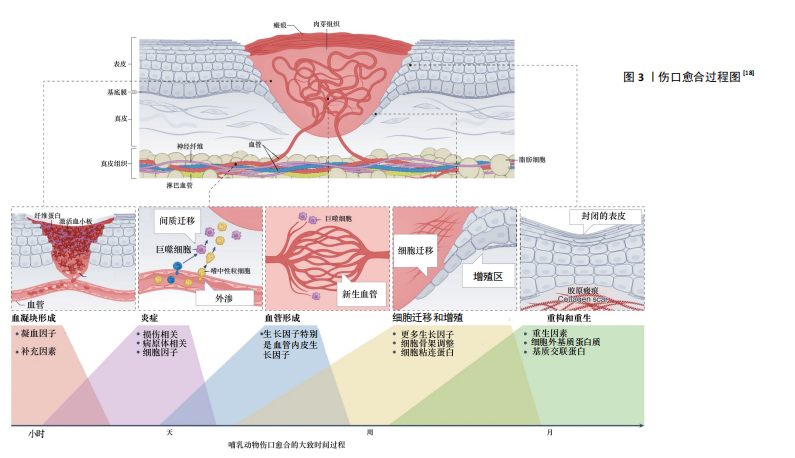
2.1 皮肤伤口愈合基本过程 正常的皮肤伤口愈合是一个动态的、复杂的、精密调控的多阶段过程,需要生长因子、细胞因子、趋化因子与各种细胞的相互协调作用[8-9]。一般将其分为凝血期、炎症期、增殖期和重塑期,这4个时期依次进行而又有一定的重叠[10]。 2.1.1 凝血期 皮肤受伤后的第一反应是止血,活化的血小板释放多种介质,如血小板源性生长因子、转化生长因子β、表皮生长因子和碱性成纤维细胞生长因子等,血小板迅速黏附在伤口上,聚集成团,为凝血激活物的集合提供了场所,开启凝血机制,能有效阻止伤口出血[11-12]。 2.1.2 炎症期 炎症在组织损伤后立即发生,通过凝血级联、炎症途径和免疫反应来减少持续的血液流失,清除坏死组织和外来异物并防止感染[13]。免疫反应涉及多种免疫细胞,如巨噬细胞、肥大细胞、淋巴细胞和中性粒细胞,这些细胞在创面处释放一系列生长因子和细胞因子,并放大凝血时释放的创面信号[14]。局部炎症反应启动之后,释放促炎递质,激活损伤相关分子模式,单核细胞到达组织损伤部位,分化为巨噬细胞,吞噬病原体并清除细胞碎片[15]。 2.1.3 增殖期 增殖期出现在皮肤损伤后的2-10 d,主要包括血管新生、肉芽组织形成和再上皮化3个阶段。血管新生是增殖过程中的关键阶段,巨噬细胞和损伤的内皮细胞释放相关生长因子,促进血管生成。新生血管可以维持创面愈合过程中的气体和营养物质的交换[16]。再上皮化是指损伤边缘角质形成细胞在表皮生长因子、转化生长因子β等生长因子刺激下增殖、迁移的过程[17]。毛细血管出芽浸润损伤部位,与成纤维细胞、免疫细胞形成肉芽组织,为细胞黏附、迁移、生长、分化提供支架。 2.1.4 重塑期 组织重塑一般发生在损伤后的两三周,表现为肉芽组织向瘢痕组织的转化,这是伤口愈合过程中的最后一个阶段。伤口再上皮化完成后,伴随着细胞迁移和凋亡,肉芽组织中各类细胞的数目逐渐减少,丰富的毛细血管网也逐渐消失,恢复至以小动脉和小静脉为主的格局。在这个阶段,多余的细胞外基质被降解,局部胶原不断更新。重塑期改善了组织的结构和强度,恢复了组织的结构和功能。 皮肤伤口愈合过程示意图[18],见图3。 2.2 皮肤中的神经 皮肤中存在复杂的神经网络,包括感觉神经和自主神经。感觉神经和自主神经都分布在皮肤上,自主神经局限于真皮,主要由交感神经组成,能够调节出汗、自主定向、血管舒张、皮肤血流量和体温稳态[7,19]。交感神经包括节前纤维和节后纤维,前者是胆碱能纤维,释放乙酰胆碱,激活M和N受体;后者是肾上腺素能纤维,主要分泌去甲肾上腺素,以及少量神经肽Y和多巴胺[20]。在物理刺激、化学刺激或者间接刺激的作用下,体外和体内的调节性神经肽、神经营养因子以及神经递质显著增加。在许多生理和病理生理条件下,自主神经起着重要的调节作用[21]。 相比之下,皮肤感觉神经在皮肤中占主导地位并广泛分布,其延伸到上表皮与环境接触[22]。感觉神经来自脑脊神经,为传入神经。皮肤的神经支配呈节段性,但相邻节段间有部分重叠。感觉神经纤维使皮肤能感受触觉、温觉、冷觉、痛觉和压觉。背根神经节神经元的末梢构成皮肤感觉神经纤维,通过脊髓将感觉器官的信号传递到大脑的感觉整合中心。真皮中源自背根神经节的皮肤感觉神经纤维可进一步分为有髓鞘的Aβ纤维和Aδ纤维,以及存在于真皮和表皮中无髓鞘的C-纤维,前者占背根神经节传入神经的80%,后者占20%[21]。在皮肤中,运动神经纤维几乎完全来自交感神经(胆碱能)神经元[23]。 2.3 神经系统在创面愈合过程中的作用 2.3.1 神经系统促进血管生成 皮肤真皮层有丰富的感觉神经支配,许多研究报道了神经及其相关分子在皮肤创面愈合过程中促进血管生成的作用。有研究表明去神经支配阻碍了伤口部位毛细血管和结缔组织的再生,通过将小鼠、大鼠或兔耳耳郭入口(或基部)的神经切除,切除无神经支配的耳廓皮肤,观察创面愈合情况,结果表明去神经支配导致伤口愈合率明显下降[24]。YU等[25]发现,糖尿病极大地扰乱了上皮、神经以及邻近细胞之间的相互依赖性,抑制了上皮细胞的增殖,引起感觉神经病变和树突状细胞密度降低,导致感觉神经再生受损、伤口"
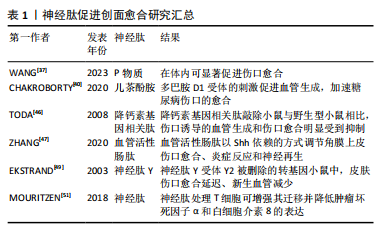
愈合明显延迟。皮肤伤口的成功愈合离不开血管活性神经肽的作用,这些神经肽扩张血管并向伤口输送血清蛋白,促进血管的再生,引起疼痛以减少进一步的损伤[26]。有报道称,相关矿物释放的Mg2+和Si4+能够促进大鼠全层皮肤创面的血管重建、上皮再生和胶原成熟,从而促进创面愈合[27]。同时,适当浓度的Mg2+通过刺激酪氨酸激酶调节神经元代谢,达到改善大鼠神经损伤的目的[28]。研究发现在人类皮肤类器官中,感觉神经支配可以改善表皮增生、促进新生血管的形成、维持肥大细胞的状态,从而证实了感觉神经支配在皮肤中的营养作用[29]。 自主神经系统调节创面愈合的各个阶段。交感神经系统以不同的方式多层次调节皮肤稳态,交感神经纤维对维持伤口周围完整皮肤的物理特性起着至关重要的作用,如果不能够进行正常的调节,可能会出现出汗引起的感染[30]。皮肤细胞产生神经肽和神经营养素,它们刺激神经纤维释放神经肽,这种相互作用形成一个积极的反馈循环,促进皮肤的炎症反应,称为神经源性炎症[31]。当出现皮肤损伤时,外周神经系统受损,损伤部位的神经过度支配,从而导致组织修复不当。神经支配的主要功能之一是增进神经元活动,刺激创面释放相关的生长因子,促进轴突发芽和轴突再生[5]。实验性去神经支配研究证实,皮肤交感神经末梢在伤口愈合中具有重要而复杂的作用,血管收缩神经元分泌去甲肾上腺素和神经肽Y,这两种物质都能减少血流量,并与炎症细胞和角质形成细胞相互作用[30]。血管生成对伤口愈合至关重要,新血管为受损组织输送氧气和营养物质,并促进碎片的清除,增加了与愈合过程中的物质代谢,交感神经可能主要通过毛细血管中的主要结构——内皮细胞和周细胞在血管生成中起作用[20]。 2.3.2 神经肽促进创面愈合 皮肤伤口愈合过程中伴随的炎症反应刺激真皮中丰富的感觉和自主神经,促进神经肽等信号的释放,这也是皮肤神经支配改善伤口愈合的主要机制。神经肽是多种神经元分泌的多肽,具有神经调节、神经递质或激素功能,属于细胞外信使家族,在细胞内通讯中发挥着非常关键的作用,感觉神经纤维和自主神经纤维分泌的神经肽在神经活动中起着重要作用[32-33]。感觉神经纤维在损伤初始阶段释放的神经肽引起血管反应,影响血流、血管通透性、血管舒缩活性或新生血管的形成;同时,神经肽释放到组织损伤环境中,对几种皮肤细胞和募集的免疫细胞进行免疫调节,从而影响受损部位组织的炎症反应。其次,神经肽还影响参与伤口愈合的各种靶细胞的增殖和分化[21]。当皮肤受伤时,神经元感知到这种破坏,并将神经递质释放到伤口微环境中,其中包括P物质、儿茶酚胺、降钙素基因相关肽和血管活性肠肽等[32,34]。 P物质是一种来自神经系统的神经肽,广泛分布于中枢和周围神经系统,主要由神经元细胞和免疫细胞产生,可以刺激血管生成和成纤维细胞增殖、促进参与伤口愈合的细胞因子和生长因子的表达,具备神经免疫调节的功能。在多种动物模型中,P物质在伤口愈合过程中被显著激活,可加速皮肤伤口愈合,并且为慢性糖尿病伤口的治疗带来了新的希望[35-36]。自主神经和感觉神经在皮肤损伤或二次暴露后,可以释放更多的P物质,在肉芽组织中,有大量的感觉神经末梢释放P物质,P物质作用于角质形成细胞和成纤维细胞,促进再上皮化愈合过程。在内皮细胞中,P物质作为血管舒张因子,能够诱导一氧化氮的产生,增强内皮细胞的通透性,促进白细胞向下层组织外渗。P物质调节多种细胞产生多种细胞因子,如促进单核细胞白细胞介素1、白细胞介素6、肿瘤坏死因子α、γ-干扰素等炎性细胞因子的合成和释放[37]。因此,P物质在伤口愈合的炎症阶段和血管生成阶段发挥至关重要的作用。 儿茶酚胺是皮肤中主要的自主神经递质,25%-50%的交感神经末梢以皮肤效应器为目标,支配血管、汗腺和毛囊,并以神经纤维的形式出现在皮肤的真皮层和表皮层[38],儿茶酚胺在维持皮肤稳态和调节炎症中发挥重要作用。严重的创伤通常作为应激源,使血液中儿茶酚胺水平迅速上升,同时刺激局部交感神经纤维放电。通常,儿茶酚胺是指去甲肾上腺素、肾上腺素和多巴胺。研究表明,在创伤后血管生成过程中,多巴胺的调控具有双重作用,这取决于所涉及的多巴胺受体的类型,血管生成是通过D1受体的激动剂激活来促进的,而多巴胺D2受体的激活对正常皮肤伤口的血管生成产生负性调节,从而延迟愈合[39-40]。多巴胺在正常和糖尿病皮肤损伤中起相反的作用,这与D1或D2受体的差异激活有关[41-42]。 降钙素基因相关肽是一种感觉神经肽,常与P物质或生长抑素在感觉神经元中共表达[43]。降钙素基因相关肽在中枢神经系统和周围神经系统中均有表达,是皮肤中表达最多的神经肽,能够表达降钙素基因相关肽的神经纤维分布于全身各个组织和器官。在伤口愈合过程中,降钙素基因相关肽在多个方面发挥作用,它能够上调血管内皮生长因子的表达,降低炎症介质如肿瘤坏死因子α的表达,减少巨噬细胞浸润,促进血管重建,并且能够增强角化细胞的增殖[43-45]。TODA等[46]表明,与WT小鼠相比,降钙素基因相关肽 KO小鼠表现出伤口诱导的血管生成缺陷和伤口愈合缺陷。 血管活性肠肽是一种具有神经调节剂和神经递质作用的神经肽,在炎症的急性期,血管活性肠肽诱导肥大细胞释放组胺和缓激肽,血管活性肠肽自身及其诱导产生的血管活性炎症递质可促进创面血管舒张,在炎症后期,血管活性肠肽可以发挥其抗炎作用[30]。此外,血管活性肠肽能够促进外周神经元的体外存活,抑制损伤后神经元细胞死亡,可能参与创面的神经再支配。有研究表明,血管活性肠肽参与调节角膜上皮伤口愈合、炎症反应和神经再生[47]。 神经肽Y是一种由36个氨基酸组成的肽,参与伤口愈合过程中炎症反应和血管生成阶段,大部分存在于中枢神经系统和周围神经系统[48]。神经肽Y具有促进血管生成的作用,并参与调节机体先天性免疫和适应性免疫。在神经肽Y-2R被删除的转基因小鼠中,可以观察到明显的皮肤伤口愈合延迟、新生血管减少的现象[49]。 神经肽主要在中枢神经系统和胃肠道中产生,神经肽下调了炎症信号转导通路核因子κB和JNK,减少了细胞因子白细胞介素6、肿瘤坏死因子α、白细胞介素10、血管内皮生长因子的表达,其下调了细胞外调节蛋白激酶信号通路,但促进了表皮生长因子的表达[50]。神经肽通过调节巨噬细胞和T细胞的功能来影响伤口愈合,用神经肽处理T细胞可增强其迁移能力并降低肿瘤坏死因子α和白细胞介素8的表达,特别的是,神经肽与P物质共刺激会导致细胞的迁移能力降低[51]。 神经肽促进创面愈合研究汇总见表1。"
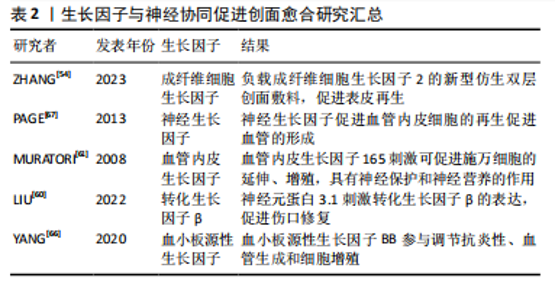
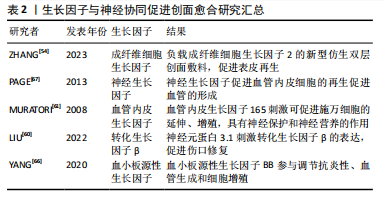
2.3.3 生长因子的协同作用 皮肤创面愈合过程受多种生长因子的调控。在伤口愈合过程中,生长因子与皮肤中不同细胞及其各自的分泌物相互作用,促进创面愈合。 成纤维细胞生长因子是一类细胞信号蛋白家族,是刺激神经分化最常用的生物因子[52]。成纤维细胞生长因子由角质细胞、成纤维细胞、内皮细胞、平滑肌细胞、软骨细胞和肥大细胞产生,成纤维细胞生长因子具有促进脊髓损伤、外周神经损伤等神经损伤修复的功能[53]。成纤维细胞生长因子可以调节炎症反应,影响免疫细胞的活性和迁移。神经肽也可以调节炎症细胞的行为,两者共同作用于伤口的炎症阶段。参与皮肤伤口愈合的主要是碱性成纤维细胞生长因子和酸性成纤维细胞生长因子。碱性成纤维细胞生长因子可以直接影响轴突,促进早期再生轴突的延长。神经和成纤维细胞生长因子共同作用可以重新诱导受损真皮中晶格状胶原纤维和成纤维细胞的形成,证明了成纤维细胞生长因子8和成纤维细胞生长因子2是潜在的皮肤再生诱导因子[6]。成纤维细胞生长因子2已被证明能够促进细胞浸润、迁移、增殖以及微血管的生成,通过制备负载成纤维细胞生长因子2的新型仿生双层创面敷料,可以促进表皮和皮肤附属器再生以及微血管生成,引入成纤维细胞生长因子2可以显著改善修复效果[54]。 神经生长因子主要由神经细胞分泌。一些研究表明,神经生长因子及相关受体参与了伤口愈合过程[55]。神经生长因子作用于神经细胞、巨噬细胞、角质形成细胞等多种细胞,能够促进成纤维细胞增殖和血管形成、影响肌成纤维细胞分化[55-57]。神经生长因子通过周围神经末梢增加神经营养因子的分泌,包括降钙素基因相关肽,从而调节与伤口愈合过程相关的免疫炎症反应。神经生长因子也被认为是一种新的血管生成分子,通过促进血管内皮细胞的再生促进血管的形成[58]。不同类型的细胞,如神经细胞、内皮细胞、肥大细胞、巨噬细胞、中性粒细胞、角质形成细胞和成纤维细胞,都通过大量的细胞因子和生长因子包括神经生长因子发挥作用,神经生长因子还通过 TrkA-PI3K/Akt 通路刺激角质形成细胞增殖和神经突延伸[59]。 血管内皮生长因子具有促进血管内皮细胞增殖、血管形成、免疫调节、神经保护、组织修复等重要作用。有研究表明,神经元蛋白3.1(P311)在促进皮肤创面愈合中起着至关重要的作用,P311能够通过增加体外和体内血管内皮生长因子的产生来增强间充质干细胞的促血管生成能力[60]。在伤口愈合的早期阶段,血管内皮生长因子的释放可以促进神经末梢的再生,而神经细胞释放的一些因子,如神经生长因子,也可以与血管内皮生长因子相互作用,共同促进创伤部位血管化以及神经再生。血管内皮生长因子165可促进施万细胞的延伸、增殖,这是促进神经突生长的一个主要过程,血管内皮生长因子还具有一些神经保护和神经营养的作用,能够促进神经元和星形胶质细胞的存活,从而表明血管内皮生长因子可能与皮肤创面愈合有很大的联系[61]。 转化生长因子β包含众多的生长因子,参与调节细胞生长、增殖、分化、迁移和凋亡等过程。肌成纤维细胞主要来源于真皮成纤维细胞的原位分化,通常由转化生长因子β信号通路启动,在创面愈合的过程中,活化的成纤维细胞增殖和巨噬细胞浸润可以产生大量的转化生长因子β[62-63]。P311通过刺激转化生长因子β的表达,促进伤口修复[60]。转化生长因子β通过激活特定的细胞信号通路,如Smad通路,影响细胞的行为,神经系统通过影响这些信号通路的活性,实现与转化生长因子β协同作用。创伤激活的神经胶质细胞,通过旁分泌调节转化生长因子β信号传导进而促进肌成纤维细胞分化[5]。转化生长因子β可以通过诱导表皮中白细胞介素31的表达,增强感觉神经元的敏感性[64]。瘢痕形成是创面愈合过程中的一个组成部分,目前很多的治疗策略都是以成纤维细胞和肌成纤维细胞中的转化生长因子β信号通路为目标,通过干扰其功能减少细胞增殖,从而减少瘢痕形成[65]。 血小板源性生长因子由同或异二聚体生长因子家族组成,包括血小板源性生长因子AA、血小板源性生长因子AB、血小板源性生长因子BB、血小板源性生长因子CC和血小板源性生长因子DD。血小板源性生长因子与血管再生密切相关,同时可以促进神经细胞存活和分化,协同促进创面修复。在损伤区域,血小板源性生长因子BB是一种有效的趋化因子,能够促进成纤维细胞、角化细胞和血管内皮细胞的分裂。还有发现指出血小板源性生长因子可以促进巨噬细胞产生和分泌生长因子,如转化生长因子β。有研究在SD大鼠背部皮肤评估了聚多巴胺辅助固定化血小板源性生长因子BB的SDGA纳米纤维基质的伤口愈合效果,结果表明血小板源性生长因子BB显著提高了转化生长因子β和血管内皮生长因子的水平,该物质通过调节抗炎性、血管生成和细胞增殖,对伤口愈合表现出极大的治疗作用[66]。 生长因子与神经协同促进创面愈合研究汇总见表2。"
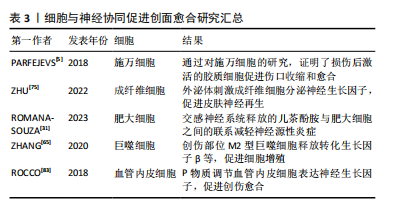
2.3.4 细胞的协同作用 皮肤神经纤维可以通过直接接触皮肤细胞,如角质形成细胞、成纤维细胞和血管内皮细胞,来调节伤口愈合和维持皮肤稳态[67-68]。 外周神经胶质细胞在神经支配伤口愈合过程中起着至关重要的作用。皮肤损伤后,被激活的胶质细胞上调与伤口愈合相关的因子的表达,并通过旁分泌调节转化生长因子β信号传导促进肌成纤维细胞分化,从而促进伤口愈合[5]。施万细胞是周围神经系统中的神经胶质细胞,皮肤中的施万细胞是一种神经嵴细胞的亚群,能调节其他细胞群体的行为,如增殖和迁移。PARFEJEVS等[5]为了明确周围神经胶质细胞在皮肤伤口愈合中的作用,使用小鼠遗传模型,采用体内特异性施万细胞谱系追踪技术,经过去分化和扩张过程,证明了损伤后激活的胶质细胞促进伤口收缩和愈合,这一过程是由促进转化生长因子β信号传导的相关因子的分泌介导的,从而导致肌成纤维细胞形成增加。研究发现,在创伤修复的早期阶段,Wnt信号上调,施万细胞去分化,在小鼠和人的糖尿病伤口中均发现施万细胞去分化缺陷,而施万细胞的缺失可能会导致产生慢性伤口,也就是说伤口部位Wnt信号下调会抑制施万细胞去分化,导致伤口修复缺陷[69]。皮肤损伤可以激活施万细胞,活化的施万细胞可以支持表皮神经纤维的再生,还可以促进血管内皮细胞的迁移[70]。将施万细胞植入组织工程支架,在体内和体外都能表达促血管生成因子,从而通过预血管化改善神经再生的效果[71]。 皮肤成纤维细胞在创面愈合过程中可以引起创面的收缩,同时促进生长因子的释放[72]。在伤口愈合过程中,成纤维细胞与再生神经紧密接触,引导轴突再生,并参与神经组织碎片的初步清除,在局部损伤后的神经再生中发挥了新的作用[73]。成纤维细胞在伤口愈合过程中与再生神经紧密接触,具有强烈的相互作用。皮肤受伤后,成纤维细胞被激活并重新编程,进一步与局部神经直接接触,有研究表明,通过 ID1/ID3-Itga6 通路促进轴突再生和伤口愈合[74]。脐带间充质干细胞外泌体可以通过招募成纤维细胞,刺激成纤维细胞分泌神经生长因子,促进皮肤神经再生,从而促进伤口愈合和皮肤再生[75]。神经PAS结构域蛋白2在成纤维细胞衰老过程中出现独特的上调,在成纤维细胞中诱导神经PAS结构域蛋白2表达可以干扰皮肤稳态、创面愈合和真皮组织重建,为治疗提供新的靶点和策略[76-77]。 皮肤中含有多种不同的干细胞,包括表皮干细胞、真皮干细胞等,不同的干细胞群体浸没在外周神经所组成的微环境中,维持局部组织的稳态,并表现出广泛的功能异质性,使皮肤具有卓越的再生能力[67]。间充质干细胞通过旁分泌作用分泌外泌体,外泌体与miRNA的结合可能是促进创伤愈合的新方法[78]。当皮肤受到损伤时,表皮和毛囊中的干细胞迁移到受损部位促进表皮的重建,而手术去除神经支配后,表皮干细胞将会丧失快速迁移的能力。炎症信号和神经递质可以促进骨髓或其他组织中的干细胞动员,使其迁移到伤口部位。到达伤口部位的干细胞受到局部微环境中的信号影响,如神经生长因子、血管内皮生长因子和其他细胞因子等,使干细胞分化为所需的细胞类型,如成纤维细胞、内皮细胞和神经细胞。目前,不同的神经纤维和各种皮肤干细胞群之间的相互作用途径尚不清楚,不同类型的感觉神经是否在干细胞调节中发挥不同的功能,将多种外部刺激与皮肤组织再生结合起来还需要深入的研究[79]。 此外,血管内皮细胞、免疫细胞、角质形成细胞等也参与创伤愈合和神经修复过程。在应激条件下,儿茶酚胺激活肥大细胞分泌抗炎细胞因子,使炎症期延迟和伤口愈合,也就说明交感神经系统释放的儿茶酚胺与肥大细胞之间的联系有助于减缓皮肤伤口愈合过程中神经源性炎症的慢性应激不良反应[31]。神经系统通过释放神经递质和神经肽与免疫系统进行交互,从而影响免疫细胞的活性和迁移,调控伤口处的细胞行为,例如影响成纤维细胞、角质形成细胞等细胞的增殖和迁移[80]。在皮肤中,感觉神经纤维和肥大细胞之间的串扰是神经源性炎症的主要机制,这对伤口的正常愈合过程至关重要[81]。体外研究表明,4-氨基吡啶可以诱导神经生长因子,增强人角质形成细胞的增殖和迁移[82]。创伤部位可以募集巨噬细胞清理碎片,M2型巨噬细胞释放生长因子,如血管内皮生长因子、血小板源性生长因子和转化生长因子β,促进细胞进入增殖期[65]。P物质调节血管内皮细胞表达神经生长因子,从而促进创伤的修复[83]。血管内皮细胞在移植微环境中转化后能够表达神经生长因子,有利于皮肤神经的再生[71]。 细胞与神经协同促进创面愈合研究汇总见表3。"
| [1] WENG T, WU P, ZHANG W, et al. Regeneration of skin appendages and nerves: current status and further challenges. J Transl Med. 2020;18(1):53. [2] DING JY, CHEN MJ, WU LF, et al. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: roles, opportunities and challenges. Mil Med Res. 2023;10(1):36. [3] BORALDI F, LOFARO FD, BONACORSI S, et al. The Role of Fibroblasts in Skin Homeostasis and Repair. Biomedicines. 2024;12(7):1586. [4] WU S, SUN S, FU W, et al. The Role and Prospects of Mesenchymal Stem Cells in Skin Repair and Regeneration. Biomedicines. 2024;12(4):743. [5] PARFEJEVS V, DEBBACHE J, SHAKHOVA O, et al. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat Commun. 2018; 9(1):236. [6] KASHIMOTO R, KAMEI Y, NONAKA S, et al. FGF signaling induces the regeneration of collagen fiber structure during skin wound healing in axolotls. Dev Biol. 2023;498:14-25. [7] CHERET J, LEBONVALLET N, CARRE JL, et al. Role of neuropeptides, neurotrophins, and neurohormones in skin wound healing. Wound Repair Regen. 2013;21(6):772-788. [8] CHOUHAN D, DEY N, BHARDWAJ N, et al. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials. 2019;216:119267. [9] ZHONG J, WANG H, YANG K, et al. Reversibly immortalized keratinocytes (iKera) facilitate re-epithelization and skin wound healing: Potential applications in cell-based skin tissue engineering. Bioact Mater. 2022;9: 523-540. [10] 肖梓腾. 外泌体与皮肤创伤的修复[J] 中国组织工程研究,2023,28(19): 3105-3109. [11] YIN J, ZHANG S, YANG C, et al. Mechanotransduction in skin wound healing and scar formation: Potential therapeutic targets for controlling hypertrophic scarring. Front Immunol. 2022;13:1028410. [12] SMANDRI A, NORDIN A, HWEI NM, et al. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers (Basel). 2020;12(8):1782. [13] GURTNER GC, WERNER S, BARRANDON Y, et al. Wound repair and regeneration. Nature. 2008;453(7193):314-321. [14] LUO R, DAI J, ZHANG J, et al. Accelerated Skin Wound Healing by Electrical Stimulation. Adv Healthc Mater. 2021;10(16):e2100557. [15] KOH TJ, DIPIETRO LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. [16] KOIKE Y, YOZAKI M, UTANI A, et al. Fibroblast growth factor 2 accelerates the epithelial-mesenchymal transition in keratinocytes during wound healing process. Sci Rep. 2020;10(1):18545. [17] MARTIN P. Wound healing-aiming for perfect skin regeneration. Science. 2016;276(5309):75-81. [18] PENA OA, MARTIN P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25(8):599-616. [19] ABDO H, CALVO-ENRIQUE L, LOPEZ JM, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365(6454):695-699. [20] PAN LL, TANG JB, LIU HW, et al. Sympathetic nerves: How do they affect angiogenesis, particularly during wound healing of soft tissues? Clin Hemorheol Micro. 2016;62(2):181-191. [21] ROOSTERMAN D, GOERGE T, SCHNEIDER SW, et al. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86(4):1309-1379. [22] NOWAK NC, MENICHELLA DM, MILLER R, et al. Cutaneous innervation in impaired diabetic wound healing. Transl Res. 2021;236:87-108. [23] LAVERDET B, DANIGO A, GIRARD D, et al. Skin innervation: important roles during normal and pathological cutaneous repair. Histol Histopathol. 2015;30(8):875-892. [24] ALAPURE BV, LU Y, PENG HY, et al. Surgical Denervation of Specific Cutaneous Nerves Impedes Excisional Wound Healing of Small Animal Ear Pinnae. Mol Neurobiol. 2018;55(2):1236-1243. [25] YU FSX, LEE PSY, YANG LL, et al. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog Retin Eye Res. 2022;89:101039. [26] CRUISE BA, XU P, HALL AK. Wounds increase activin in skin and a vasoactive neuropeptide in sensory ganglia. Dev Biol. 2004;271(1):1-10. [27] MEHRABI T, MESGAR AS, MOHAMMADI Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater Sci Eng. 2020;6(10):5399-5430. [28] ZHUANG P, YAO Y, SU X, et al. Vascularization and neuralization of bioactive calcium magnesium phosphate/hydrogels for wound healing. Compos Part B Eng. 2022;242:110030. [29] CHÉRET J, PICCINI I, GHERARDINI J, et al. Sensory Reinnervation of Human Skin by Human Neural Stem Cell-Derived Peripheral Neurons Ex Vivo. J Invest Dermatol. 2022;142(1):257-261.e5. [30] IVANOV E, AKHMETSHINA M, ERDIAKOV A, et al. Sympathetic System in Wound Healing: Multistage Control in Normal and Diabetic Skin. Int J Mol Sci. 2023;24(3):2045. [31] ROMANA-SOUZA B, CHEN L, DIPIETRO LA. Repeated stress-induced crosstalk between the sympathetic nervous system and mast cells contributes to delayed cutaneous wound healing in mice. J Neuroimmunol. 2023;379:578104. [32] GUPTA D, KAUSHIK D, MOHAN V. Role of neurotransmitters in the regulation of cutaneous wound healing. Exp Brain Res. 2022;240(6):1649-1659. [33] ASHRAFI M, BAGUNEID M, BAYAT A. The Role of Neuromediators and Innervation in Cutaneous Wound Healing. Acta Derm-Venereol. 2016;96(5): 587-594. [34] KIRCHNER S, LEI V, MACLEOD AS. The Cutaneous Wound Innate Immunological Microenvironment. Int J Mol Sci. 2020;21(22):8748. [35] KANT V, KUMAR D, KUMAR D, et al. Topical application of substance P promotes wound healing in streptozotocin-induced diabetic rats. Cytokine. 2015;73(1):144-155.
[36] LI H, LI MN, LIU P, et al. A multifunctional substance P-conjugated chitosan hydrochloride hydrogel accelerates full-thickness wound healing by enhancing synchronized vascularization, extracellular matrix deposition, and nerve regeneration. Biomater Sci-Uk. 2021;9(11):4199-4210. [37] WANG XX, WU JL, WANG M, et al. Substance P&dimethyloxallyl glycine-loaded carboxymethyl chitosan/gelatin hydrogel for wound healing. J Biomed Mater Res A. 2023;111(3):404-414. [38] BORREL V, THOMAS P, CATOVIC C, et al. Acne and Stress: Impact of Catecholamines on Cutibacterium acnes. Front Med (Lausanne). 2019;6:155. [39] VAUGHN AR, DAVIS MJ, SIVAMANI RK, et al. A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing. Molecules. 2018;23(1):50. [40] CHAKROBORTY D, GOSWAMI S, BASU S, et al. Catecholamines in the regulation of angiogenesis in cutaneous wound healing. Faseb J. 2020; 34(11):14093-14102. [41] CHAKROBORTY D, SARKAR C, LU K, et al. Activation of Dopamine D Receptors in Dermal Fibroblasts Restores Vascular Endothelial Growth Factor-A Production by These Cells and Subsequent Angiogenesis in Diabetic Cutaneous Wound Tissues. Am J Pathol. 2016;186(9):2262-2270. [42] SHOME S, RANA T, GANGULY S, et al. Dopamine Regulates Angiogenesis in Normal Dermal Wound Tissues. Plos One. 2011;6(9):e25215. [43] GRANSTEIN RD, WAGNER JA, STOHL LL, et al. Calcitonin gene-related peptide: key regulator of cutaneous immunity. Acta Physiol. 2015;213(3): 586-594. [44] WURTHMANN S, NÄGEL S, HADASCHIK E, et al. Impaired wound healing in a migraine patient as a possible side effect of calcitonin gene-related peptide receptor antibody treatment: A case report. Cephalalgia. 2020;40(11): 1255-1260. [45] ROGGENKAMP D, KÖPNICK S, STÄB F, et al. Epidermal Nerve Fibers Modulate Keratinocyte Growth via Neuropeptide Signaling in an Innervated Skin Model. J Invest Dermatol. 2013;133(6):1620-1628. [46] TODA M, SUZUKI T, HOSONO K, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62(6):352-359. [47] ZHANG YY, GAO N, WU L, et al. Role of VIP and Sonic Hedgehog Signaling Pathways in Mediating Epithelial Wound Healing, Sensory Nerve Regeneration, and Their Defects in Diabetic Corneas. Diabetes. 2020; 69(7):1549-1561. [48] THEOCHARIDIS G, VEVES A. Autonomic nerve dysfunction and impaired diabetic wound healing: The role of neuropeptides. Auton Neurosci-Basic. 2020;223:102610. [49] EKSTRAND AJ, CAO R, BJORNDAHL M, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. PNAS. 2003;100(10):6033-6038. [50] LIU JH, YAN LW, YANG W, et al. Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact Mater. 2019;4:151-159. [51] MOURITZEN MV, ABOURAYALE S, EJAZ R, et al. Neurotensin, substance P, and insulin enhance cell migration. J Pept Sci. 2018;24(7):e3093. [52] CHEN SQ, CAI Q, SHEN YY, et al. Combined use of NGF/BDNF/bFGF promotes proliferation and differentiation of neural stem cells in vitro. Int J Dev Neurosci. 2014;38:74-78. [53] ZUBAIR M, AHMAD J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Dis. 2019;20(2):207-217. [54] ZHANG MM, XU SX, DU C, et al. Novel PLCL nanofibrous/keratin hydrogel bilayer wound dressing for skin wound repair. Colloid Surface B. 2023:222: 113119. [55] EL BAASSIRI MG, DOSH L, HAIDAR H, et al. Nerve growth factor and burn wound healing: Update of molecular interactions with skin cells. Burns. 2023;49(5):989-1002. [56] TROULLINAKI M, ALEXAKI VI, MITROULIS I, et al. Nerve growth factor regulates endothelial cell survival and pathological retinal angiogenesis. J Cell Mol Med. 2019;23(4):2362-2371. [57] LIU ZX, CAO YQ, LIU GJ, et al. p75 neurotrophin receptor regulates NGF-induced myofibroblast differentiation and collagen synthesis through MRTF-A. Exp Cell Res. 2019;383(1):111504. [58] Gostynska N, Pannella M, Rocco ML, et al. The pleiotropic molecule NGF regulates the in vitro properties of fibroblasts, keratinocytes and endothelial cells: implications for wound healing. Am J Physiol Cell Physiol. 2020;318(2):C360-C371. [59] G EL BAASSIRI M, DOSH L, HAIDAR H, et al. Nerve growth factor and burn wound healing: Update of molecular interactions with skin cells. Burns. 2023;49(5):989-1002. [60] LIU ZH, YANG JC, CHEN YX, et al. P311 Facilitates the Angiogenesis and Wound Healing Function of MSCs by Increasing VEGF Production. Front Immunol. 2022;13:821932. [61] MURATORI L, GNAVI S, FREGNAN F, et al. Evaluation of Vascular Endothelial Growth Factor (VEGF) and Its Family Member Expression After Peripheral Nerve Regeneration and Denervation. Anat Rec. 2018;301(10):1646-1656. [62] KIM KK, SHEPPARD D, CHAPMAN HA. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293. [63] ZHANG Y, PAN YJ, LIU YH, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res Ther. 2021;12(1):434. [64] XU J, ZANVIT P, HU L, et al. The Cytokine TGF-beta Induces Interleukin-31 Expression from Dermal Dendritic Cells to Activate Sensory Neurons and Stimulate Wound Itching. Immunity. 2020;53(2):371-383.e5. [65] ZHANG T, WANG XF, WANG ZC, et al. Current potential therapeutic strategies targeting the TGF-beta/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020;129:110287. [66] YANG X, ZHAN P, WANG X, et al. Polydopamine-assisted PDGF-BB immobilization on PLGA fibrous substrate enhances wound healing via regulating anti-inflammatory and cytokine secretion. Plos One. 2020;15(9):e0239366. [67] PAGE ME, LOMBARD P, NG F, et al. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13(4):471-482. [68] TALAGAS M, LEBONVALLET N, LESCHIERA R, et al. Keratinocytes Communicate with Sensory Neurons via Synaptic-like Contacts. Ann Neurol. 2020;88(6):1205-1219. [69] OU MY, TAN PC, XIE Y, et al. Dedifferentiated Schwann cell-derived TGF-β3 is essential for the neural system to promote wound healing. Theranostics. 2022;12(12):5470-5487. [70] BRAY ER, CHÉRET J, YOSIPOVITCH G, et al. Schwann cells as underestimated, major players in human skin physiology and pathology. Exp Dermatol. 2020;29(1):93-101. [71] LI MY, CHENG XY, FENG SY, et al. Skin precursor-derived Schwann cells accelerate in vivo prevascularization of tissue-engineered nerves to promote peripheral nerve regeneration. Glia. 2023;71(7):1755-1769. [72] 卓雅婷, 李许. 成纤维细胞在皮肤创伤修复中的作用研究进展[J]. 药学研究,2021,40(3):191-195. [73] ZHOU WX, RAHMAN MSU, SUN CM, et al. Perspectives on the Novel Multifunctional Nerve Guidance Conduits: From Specific Regenerative Procedures to Motor Function Rebuilding. Adv Mater. 2024;36(14):e2307805. [74] CHEN Z, SHEN G, TAN X, et al. ID1/ID3 mediate the contribution of skin fibroblasts to local nerve regeneration through Itga6 in wound repair. Stem Cells Transl Med. 2021;10(12):1637-1649. [75] ZHU ZY, ZHANG XA, HAO HJ, et al. Exosomes Derived From Umbilical Cord Mesenchymal Stem Cells Treat Cutaneous Nerve Damage and Promote Wound Healing. Front Cell Neurosci. 2022;16:913009. [76] SASAKI H, HOKUGO A, WANG L, et al. Neuronal PAS Domain 2 (Npas2)-Deficient Fibroblasts Accelerate Skin Wound Healing and Dermal Collagen Reconstruction. Anat Rec. 2020;303(6):1630-1641. [77] GLASS D, VIÑUELA A, DAVIES MN, et al. Gene expression changes with age in skin, adipose tissue, blood and brain. Genome Biol. 2013;14(7):R75. [78] JU CC, LIU DW. Exosomal microRNAs from Mesenchymal Stem Cells: Novel Therapeutic Effect in Wound Healing. Tissue Eng Regen Med. 2023;20(5): 647-660. [79] PENG JY, CHEN H, ZHANG B. Nerve-stem cell crosstalk in skin regeneration and diseases. Trends Mol Med. 2022;28(7):583-595. [80] CIOCE A, CAVANI A, CATTANI C, et al. Role of the Skin Immune System in Wound Healing. Cells. 2024;13(7):624. [81] HENDRIX S, PETERS EM. Neuronal plasticity and neuroregeneration in the skin -- the role of inflammation. J Neuroimmunol. 2007;184(1-2):113-126. [82] JAGADEESHAPRASAD MG, GOVINDAPPA PK, NELSON AM, et al. 4-Aminopyridine Induces Nerve Growth Factor to Improve Skin Wound Healing and Tissue Regeneration. Biomedicines. 2022;10(7):1649. [83] ROCCO ML, SOLIGO M, MANNI L, et al. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr Neuropharmacol. 2018;16(10):1455-1465. |
| [1] | Li Zikai, Zhang Chengcheng, Xiong Jiaying, Yang Xirui, Yang Jing, Shi Haishan. Potential effects of ornidazole on intracanal vascularization in endodontic regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-7. |
| [2] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [3] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [4] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [5] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [6] | De Ji, Suo Langda, Wei Yuchen, Wang Bin, Awangcuoji, Renqingcuomu, Cui Jiuzeng, Zhang Lei, Ba Gui. Comprehensive analysis of genes related to endometrial receptivity and alternative splicing events in northwest Tibetan cashmere goats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1429-1436. |
| [7] | Peng Hongcheng, Peng Guoxuan, Lei Anyi, Lin Yuan, Sun Hong, Ning Xu, Shang Xianwen, Deng Jin, Huang Mingzhi . Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1497-1503. |
| [8] | Chen Yuning, Jiang Ying, Liao Xiangyu, Chen Qiongjun, Xiong Liang, Liu Yue, Liu Tong. Buqi Huoxue Compounds intervene with the expression of related factors and autophagy related proteins in a rat model of cerebral ischemia/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1152-1158. |
| [9] | Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977. |
| [10] | Yang Bin, Tao Guangyi, Yang Shun, Xu Junjie, Huang Junqing . Visualization analysis of research hotspots of artificial intelligence in field of spinal cord nerve injury and repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 761-770. |
| [11] | Li Chen, Liu Ye, Ni Xindi, Zhang Yuang. Simulation analysis of real-time continuous stiffness in muscle fibers and tendons of the triceps surae during multi-joint movement [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7529-7536. |
| [12] | Yang Bo, Pan Xinfang, Chang Liuhui, Ni Yong. Correlation of echocardiographic parameters with disability at 3 months after acute ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7544-7551. |
| [13] | Liu Xuan, Ding Yuqing, Xia Ruohan, Wang Xianwang, Hu Shujuan. Exercise prevention and treatment of insulin resistance: role and molecular mechanism of Keap1/nuclear factor erythroid2-related factor 2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7578-7588. |
| [14] | Wang Xuepeng, , He Yong, . Effect of insulin-like growth factor family member levels on inflammatory arthritis: a FinnGen biobank-based analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7656-7662. |
| [15] | Gong Yuehong, Wang Mengjun, Ren Hang, Zheng Hui, Sun Jiajia, Liu Junpeng, Zhang Fei, Yang Jianhua, Hu Junping. Machine learning combined with bioinformatics screening of key genes for pulmonary fibrosis associated with cellular autophagy and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7679-7689. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||