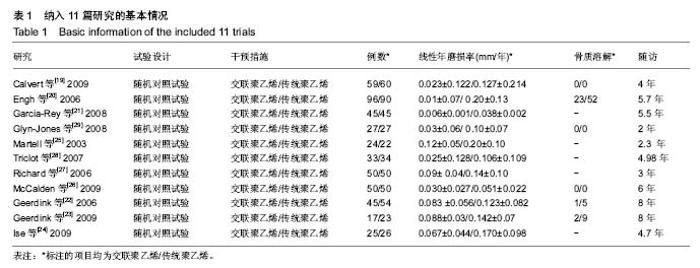
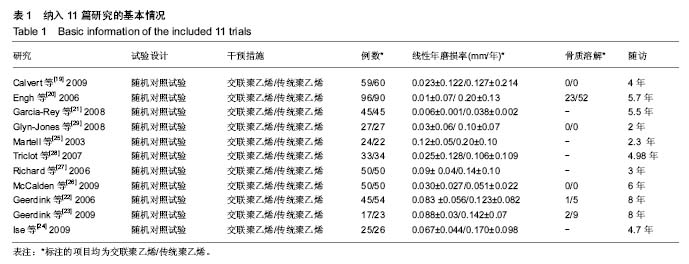
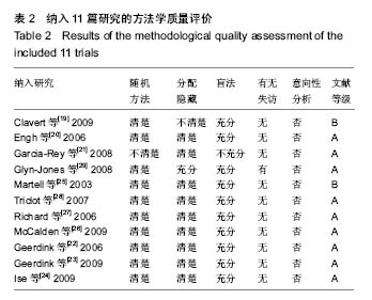
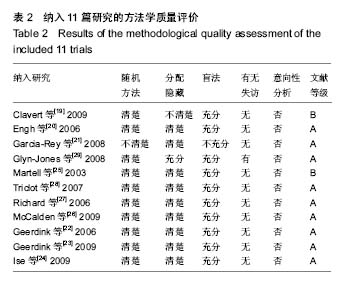
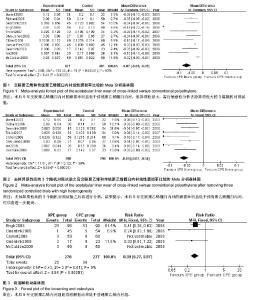
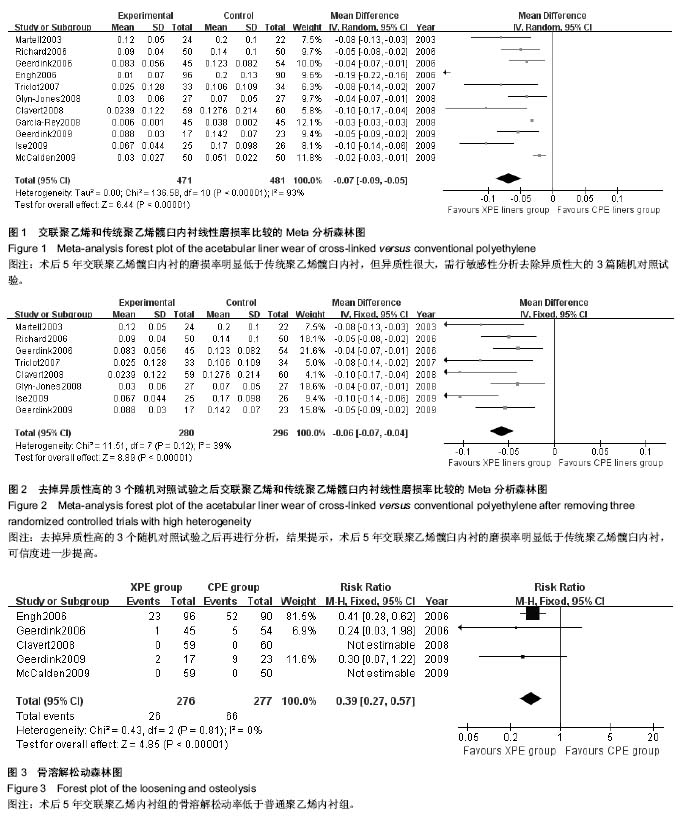
| [1] 邢金锋, 宋科官, 彭锂,等. 全关节成形术中假体周围骨溶解的概述:本体因素与未来发展方向[J]. 现代生物医学进展, 2016, 16(2): 386-389+316.[2] 蒋营军, 吴连国. 人工关节置换术后磨损颗粒与假体周围骨溶解的研究进展[J]. 中国骨伤, 2016,30(10): 968-972.[3] 杨兴, 薛峰, 盛晓文,等. 金属对聚乙烯假体全髋关节置换术后磨损的中期随访研究[J]. 岭南现代临床外科, 2015,15(1): 87-90.[4] 陈锐, 戴闽. 关节置换术后假体周围骨溶解相关病理研究[J]. 国际骨科学杂志, 2009,46(6): 379-381.[5] 许可, 马信龙, 李稚君,等. 全髋关节置换术应用高交联和传统聚乙烯内衬的Meta分析[J]. 中华创伤杂志, 2013,29(12): 1163-1169.[6] 王萍, 谢蟪旭, 张绮,等. 如何在Cochrane系统评价中设计资料提取表[J]. 中国循证医学杂志, 2011,11(3): 341-345.[7] 贾文琴,杨克虎,田金徽,等.cochrane系统评价发表状况调查[J].中国循证医学杂志,2009,9(6):635-639.[8] 李静, 李幼平. 不断完善与发展的Cochrane系统评价[J]. 中国循证医学杂志, 2008,8(9): 742-743.[9] 李静, 秦莉, 刘鸣. 系统评价的基本方法[J]. 中国循证医学, 2001,1(1): 34-38.[10] 管红珍, 彭智聪, 傅鹰. 循证医学中文献证据等级标准的系统性综述[J]. 药物流行病学杂志, 2002,11(3):145-148+169.[11] 吴泰相, 刘关键, 李静. 影响系统评价质量的主要因素浅析[J].中国循证医学杂志, 2005,5(1): 51-58.[12] 张俊华, 商洪才, 张伯礼. 系统评价和meta分析质量的评价方法[J].中西医结合学报, 2008,6(4): 337-340.[13] 钟晓蓉, 罗斌. Cochrane系统评价用户文摘(4)[J]. 华西医学, 2003,18(2):152-154.[14] 谷鸿秋, 王杨, 李卫. Cochrane偏倚风险评估工具在随机对照研究Meta分析中的应用[J]. 中国循环杂志, 2014,29(2):147-148.[15] 卫茂玲.cochrane系统评价在中国的现状与问题[J].中国循证医学杂志,2006,6(2):150-151.[16] 孙晓莹, 马婧, 赵晔,等. Cochrane系统评价现状[J]. 中国循证儿科杂志, 2013,8(3): 237-240.[17] 刘建平. 非随机研究的系统评价方法(一)[J]. 中国循证医学, 2001,1(4): 239-243.[18] 屈会起, 张金钟, 邱明才. 循证医学的系统评价方法[J]. 中华医院管理杂志, 2000,16(6): 13-15.[19] Calvert GT, Devane PA, Fielden J, et al. A double-blind, prospective, randomized controlled trial comparing highly cross-linked and conventional polyethylene in primary total hip arthroplasty. J Arthroplasty. 2009;24(4): 505-510.[20] Engh CA Jr, Stepniewski AS, Ginn SD, et al. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21(6 Suppl 2): 17-25.[21] Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A, et al. New polyethylenes in total hip replacement: a prospective, comparative clinical study of two types of liner. J Bone Joint Surg Br. 2008;90(2): 149-153.[22] Geerdink CH, Grimm B, Ramakrishnan R, et al. Crosslinked polyethylene compared to conventional polyethylene in total hip replacement: pre-clinical evaluation, in-vitro testing and prospective clinical follow-up study. Acta Orthop. 2006;77(5): 719-725.[23] Geerdink CH, Grimm B, Vencken W, et al. Cross-linked compared with historical polyethylene in THA: an 8-year clinical study. Clin Orthop Relat Res. 2009;467(4): 979-984.[24] Ise K, Kawanabe K, Tamura J, et al. Clinical results of the wear performance of cross-linked polyethylene in total hip arthroplasty: prospective randomized trial. J Arthroplasty. 2009;24(8): 1216-1220.[25] Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(7 Suppl 1): 55-59.[26] McCalden RW, MacDonald SJ, Rorabeck CH, et al. Wear rate of highly cross-linked polyethylene in total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91(4): 773-782.[27] Olyslaegers C, Defoort K, Simon JP, et al. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty. 2008;23(4): 489-494.[28] Triclot P, Grosjean G, El Masri F, et al. A comparison of the penetration rate of two polyethylene acetabular liners of different levels of cross-linking. A prospective randomised trial. J Bone Joint Surg Br. 2007;89(11): 1439-1445.[29] Glyn-Jones S, McLardy-Smith P, Gill HS, et al. The creep and wear of highly cross-linked polyethylene: a three-year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br. 2008;90(5): 556-561.[30] 胡通洲, 童培建, 肖鲁伟. 全髋关节置换后聚乙烯磨损[J]. 国际骨科学杂志, 2008,29(1): 49-51.[31] 梁材. 人工股骨头置换术后髋臼磨损的影响因素[J]. 临床医药实践, 2014,23(8): 605-608.[32] Dorr LD, Wan Z, Shahrdar C, et al. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8): 1816-1821.[33] Rajadhyaksha AD, Brotea C, Cheung Y, et al. Five-year comparative study of highly cross-linked (crossfire) and traditional polyethylene. J Arthroplasty. 2009;24(2): 161-167.[34] Ingram JH, Stone M, Fisher J, et al. The influence of molecular weight, crosslinking and counterface roughness on TNF-alpha production by macrophages in response to ultra high molecular weight polyethylene particles. Biomaterials. 2004;25(17): 3511-3522.[35] Yan Y, Chen H, Feng J, et al. Poor performance of Enduron polyethylene liner in total hip arthroplasty: a minimum ten-year follow up and ultra-morphological analysis of wear particles. Int Orthop. 2016. [Epub ahead of print][36] Bradford L, Baker D, Ries MD, et al. Fatigue crack propagation resistance of highly crosslinked polyethylene. Clin Orthop Relat Res. 2004;(429): 68-72.[37] Bradford L, Baker DA, Graham J, et al. Wear and surface cracking in early retrieved highly cross-linked polyethylene acetabular liners. J Bone Joint Surg Am. 2004;86-A(6): 1271-1282.[38] Muratoglu OK, Wannomae K, Christensen S, et al. Ex vivo wear of conventional and cross-linked polyethylene acetabular liners. Clin Orthop Relat Res. 2005;438: 158-164.[39] 张国强, 王岩, 陈继营,等. 陶瓷对聚乙烯和金属对聚乙烯在全髋关节置换术后磨损的随访研究[J]. 中华关节外科杂志(电子版), 2010,4(3): 305-309. |