Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (11): 1790-1797.doi: 10.3969/j.issn.2095-4344.2017.11.026
Previous Articles Next Articles
Recent advances in vivo model of lumbar disc degeneration
Ma Sheng, Jia Yu-song, Sun Qi
- First Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China
-
Online:2017-04-18Published:2017-05-06 -
Contact:Sun Qi, M.D., Associate chief physician, First Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China -
About author:Ma Sheng, Studying for master’s degree, First Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China -
Supported by:the Project of Natural Science Research in Universities Directly under the Ministry of Education, No. 2015-JYB-JSMS065
CLC Number:
Cite this article
Ma Sheng, Jia Yu-song, Sun Qi. Recent advances in vivo model of lumbar disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(11): 1790-1797.
share this article

2.1 物理方式诱导 2.1.1 脊柱失稳法 脊柱失稳法通常需要在手术操作基础上完成。张昭等[2]通过切除腰椎棘上及棘间韧带,并咬除椎体两侧下关节突,造成椎间失稳,成功构建椎间盘退变动物模型。Phillips等[3]用不锈钢丝连接固定5,6,7横突,用甲基丙烯酸甲酯包围横突,骨水泥固化后缝合伤口。3个月后,邻近椎间隙出现轻微变窄,9个月后,邻近椎间隙狭窄程度接近50%,并出现了椎体终板硬化以及骨赘形成等退行性改变。脊柱的融合造成脊柱的正常应力传导情况发生改变,从而造成相邻节段椎间盘的应力明显增加,最终导致了邻近节段椎间盘因失稳而退变。该造模法符合人椎间盘退变的客观规律,但是手术操作稍显繁琐,创伤较大,血运破坏大,对所观察椎间盘可能形成干扰,不利于对椎间盘退变的防治进行深入研究。而且构建脊柱失稳性椎间盘退变动物模型时,诱发椎间盘退变所需的时间不一,同时建模过程中脊柱可能逐渐发生纤维化而稳定,进而影响建模。 脊柱动、静力平衡失调(椎间不稳),加上异常应力刺激,可稳定地诱发出兔腰椎间盘退变模型。Zhang等[4]在研究β1整合素通过ERK1/2 MAPK通路抑制纤维环细胞周期性拉伸诱导的凋亡时,切除实验组大鼠脊柱元件,包括脊旁肌、韧带以及小关节,破坏腰椎的稳定性。术后6,12和18周行X射线及MRI检查,经检查可见实验组手术节段椎间隙变窄,终板钙化,MRI的T2加权像出现低信号,即发生椎间盘组织脱水、蛋白多糖含量减少、硫酸软骨素与角质素比率下降等改变。实验结果显示此方法造成的退变主要表现为纤维环断裂,髓核向后突出,终板软骨钙化层增厚,透明软骨变薄及软骨细胞坏死等,此方法能否导致椎体骨质的增生还需要长期的观察和更精细的实验设计。 2.1.2 穿刺法 针刺损伤纤维环的动物模型能够模拟人类纤维环损伤、髓核突出以及退变的过程。Lipson等[5]的刺伤模型被奉为穿刺造模研究退变的经典之作,11号刀片经腹侧全层刺伤纤维环,产生组织学及生化的改变,但刀片损伤纤维环全层后,髓核即刻突出,不适合研究慢性退变的过程。Sun等[6]采用兔作为模型,通过前外侧腹膜后入路暴露兔脊柱,用16号注射针对新西兰兔L4-5间盘进行深度5 mm的环状穿刺,结果显示L4-5椎间盘退变程度逐渐增加,并且在第3周退变速度达到最快,3周后退变速度减缓,从而成功制作腰椎间盘退变动物模型。崔运能等[7]将兔剪断L5、L6右侧横突后暴露椎间盘并用18 G针穿刺构建模型,结果显示,4周后实验组L4/5、L5/6椎间盘高度明显降低、骨赘形成、终板硬化,MRI的T2加权像亦见椎间盘高度下降,其椎间盘髓核信号明显减弱;组织学观察髓核脊索细胞数量减少,免疫组织化学观察到髓核Ⅱ型胶原表达比对照组和假手术组明显减少。从而提供了一种理想的、相对可靠的腰椎间盘退变的实验动物模型,详见图1,2。Obata 等[8]在研究自体富血小板血浆释放对兔环状穿刺椎间盘退变的影响时,采用兔进行环状穿刺诱导椎间盘退变模型。Mwale等[9]在研究Link N作为一种修复介质在兔子椎间盘退变中的疗效观察时,也采用了同样的方法。 另外,Oehme等[10]在研究通过STRO-3-阳性同种异体间质前体细胞与戊聚糖多硫酸盐来重建退变的羊腰椎间盘时,对24只羊分别在L2-3,L3-4,L4-5处做环形切口,从而成功诱导椎间盘变性。Chen等[11]在研究定量T2驰豫时间和磁转移比预测腰椎间盘退变犬模型中终板生化含量时,对16只狗的腰椎间盘行环形穿刺诱导退变,经生化和组织学分析,其含量与退化的MRI信号强度成相关性。选用绵羊或狗取代常用的家兔模型,因为前两者的脊柱与人类脊柱在解剖及结构上很相似,用来制作腰椎退变模型更符合实际情况,也更具有临床研究意义。 由此可见穿刺法可通过损伤椎间盘纤维环,从而诱导椎间盘退变,此方法实验条件要求较低,操作相对简单,可重复性强,但观察周期相对较长,且评价方法是视觉的,主观的,仅凭信号强度和形态的逻辑变化来评判椎间盘退变的程度可能存在观察者的偏倚,尤其是当信号强度或形态的变化相对较小时,其评价往往存在主观色彩。黄河等[12]比较纤维环穿刺法与腰椎失稳法构建椎间盘退变模型,发现纤维环穿刺法较腰椎失稳法椎间盘退变出现的早、进展快且程度严重。 2.1.3 改变脊柱负荷 脊柱的异常应力可直接损伤椎间盘的结构。首先,在轴向压力的作用下,椎体软骨终板、骨性终板及终板下骨小粱发生弯曲变形;其次,长时间的异常应力作用可促使终板软骨细胞合成代谢减少,分解代谢增加,其中硫酸软骨素的含量急剧减少会引起终板钙化,化学成分的改变继发形态及功能的变化。生物力学性能改变及椎间盘营养物质交换通路的破坏,诱发和促进了椎间盘退变的发生。可见,力学因素在椎间盘退变过程中的作用显得尤为突出,这成为改变脊柱负荷来建造腰椎退变动物模型的重要理论支撑。 Guehring等[13]在关于不同应力状态下椎间盘内压力测定的研究结果表明,椎间盘内压力随节段不同而各自不同,短暂压力负荷可减少椎间盘内压力,持久的外在压力可导致椎间盘内水分的丢失以及蛋白变性,从而引起椎间盘的退变。Guehring等[14]同样采用了压缩椎间盘的方法,在加载负荷后发现终板细胞标志物降低、终板硬化及纤维化增加。实验表明脊索细胞不能抵御机械应力,加载负荷后有限的营养不容易通过结构改变后的终板,从而诱导椎间盘退变,详见图3,4。Lindblom[15]用鼠尾“折弯法”构建尾椎椎间盘退变模型已有55年历史。Wertz等[16]使用动态压缩法对大鼠尾椎进行椎间盘退变造模实验,当施加过度的持续时间动态压缩情况下,大鼠尾椎发生类似人类椎间盘退变的缓慢渐进过程,这比静态压缩或弯曲造模能产生更显著的基因变化和组织结构破坏。 2.1.4 椎体终板损伤法 椎体的软骨终板是供应椎间盘营养的重要通道,椎间盘赖以发挥生理机能的各种营养成分诸如水、胶原及蛋白多糖等都由此通道传递[17-18]。然而椎体终板又是脊柱运动单元中最容易受损伤的部位,异常应力造成的过度轴向负荷可导致椎体软骨终板、骨性终板及终板下骨小梁弯曲变形,从而造成软骨终板与骨性终板的分离。Shirazi-Adl等[19]认为软骨终板渗透性的中断或损伤都会影响营养物质的扩散及分布,养分含量可能会下降到不足以维持细胞活性的水平,最终导致细胞死亡和椎间盘退变。Sobajimas等[20]同样认为营养障碍是引发椎间盘退变的主要原因。因此,椎体终板损伤从而造成的椎间盘营养供应障碍也会导致椎间盘退变。 Cinotti等[21]通过对猪腰椎间盘软骨终板损伤模型进行检测发现,椎间盘内的细胞成分、蛋白多糖及水分均出现不同程度减少,说明损伤椎体终板可导致猪椎间盘退变,而且退变程度与损伤程度有关。然而由于破坏终板程度不易掌握,损伤后可立即出现一些变化,如Schmorl结节、局部血肿及周围瘢痕组织形成等,均会对后续实验及观察造成影响。Holm等[22]对6头家猪的L4椎体颅侧终板进行穿孔,3个月后观察发现实验组椎间盘外侧纤维环含水量与髓核蛋白多糖明显减少,同时椎间盘内的压力明显降低,细胞成分也发生不同程度减少,髓核失去应有的果冻样结构,纤维环出现分层,从而验证椎体终板的损伤是造成椎间盘退变的重要原因之一。Cinotti等[21]还对10头家猪的腰椎进行了另一项椎体终板损伤的研究,结果发现椎体终板损伤严重者,其相应的椎间盘退变也明显,而单一椎体终板损伤者只是相应的椎间盘发生退变。吕浩然等[23]用特制弧形针损伤兔腰椎椎体终板,发现终板损伤法可获得缓慢退变的椎间盘退变模型,终板损伤模型比纤维环损伤模型更容易出现纤维环退行性改变,但终板的破坏程度不易控制。 2.2 化学方式诱导 化学方式诱导是通过化学试剂损伤的方式,将药物注入椎间盘内,选择性破坏椎间盘内的蛋白多糖,造成髓核蛋白黏稠度下降,纤维环层状结构疏松,椎间盘的细胞形态发生改变,继而造成脊柱的生物力学稳定性降低,最终诱导椎间盘的退变。椎间盘退变是椎间盘内组织进行性结构丧失的一种异常表现,该过程是由细胞介导的应答反应,其内部结构和成分变化导致力学功能异常。应用化学制剂诱导椎间盘退变具有重复性强,操作相对简单等优点,是目前腰椎退变造模的常用方法之一。 2.2.1 木瓜蛋白酶 Sato等[24]在研究椎间盘内注射高渗盐水对椎间盘内压力的影响时采用兔作为实验对象,结果表明,椎间盘内注射高渗生理盐水会暂时降低椎间盘内压力,而注射木瓜蛋白酶则会造成椎间盘内压力永久降低,以此可建立腰椎间盘退变模型。胶原酶溶解术是由Hirsch[25]首先提出的治疗腰痛和坐骨神经痛的方案,并由Smith[26]第一次将木瓜蛋白酶应用于临床,其机制是快速水解椎间盘内的蛋白多糖,造成髓核蛋白黏稠度下降,纤维环层状排列松弛,椎间盘细胞形态发生改变,并导致脊柱生物力学稳定性降低[27]。通过影像学检查可发现椎间盘高度降低。然而该酶毒性较强,且经常遇到过敏反应等一些棘手的并发症,因此,它渐渐被其他更安全有效的酶类取代。 2.2.2 白细胞介素1β 白细胞介素1β可通过影响基质金属蛋白酶的生物活性以及抑制基质中蛋白多糖的合成来参与椎间盘的退变。颜登鲁等[28]在兔椎间盘内注射白细胞介素1β,用苏木精-伊红染色在偏差显微镜下进行椎间盘组织观察,并用透射电镜观察椎间盘组织超微结构。实验结果表明白细胞介素1β作用下腰椎间盘退变动物模型与外力作用下腰椎间盘退变动物模型相似,成功建立了白细胞介素1β作用下的腰椎退变动物模型。 2.2.3 胰蛋白酶 Omlor等[29]采用胰蛋白酶对猪腰椎间盘进行注射,通过比较3周及24周后髓核的重量来验证胰蛋白酶制作腰椎间盘退变模型的可行性。Guder等[30]同样采用胰蛋白酶对羊成功造模,并发现了48周后处死的羊腰椎间盘退变程度较12周组更明显。 2.2.4 软骨素酶ABC Hoogendoorn等[31]采用软骨素酶注射于山羊腰椎间隙,记录注射后4,8,12,18和26周椎间盘的质量,并结合影像学及生物力学的结果分析,成功制造山羊腰椎间盘退变模型,此方法毒性小且作用显著,常用于大型动物模型的制作。Norcross等[32]以大鼠作为实验模型,并用软骨素酶ABC注入其椎间盘内,2周后便能观察到实验组椎间盘质量减轻,蛋白聚糖含量减少,髓核细胞减少等一系列退变表现。Peeters等[33]在研究轻微退变的腰椎间盘中骨形态发生蛋白2及骨形态发生蛋白2/7与纤维蛋白/透明质酸水凝胶的偶联关系时,通过注射软骨素酶ABC,在7只成年荷兰奶山羊的5个腰椎间盘诱导了轻度退化。 Boxberger等[34]采用软骨素酶ABC注入实验组大鼠腰椎间盘髓核中,4周后显示实验组与对照组相比,髓核黏多糖减少,椎间盘质量减轻,力学参数中拉伸与压缩系数下降;12周后各项参考指标较4周前均有所回升。实验结果表明,软骨素酶可降解椎间盘内的蛋白聚糖,使椎间盘发生退变,但不能破坏椎间盘基质的再生能力,当软骨素酶浓度降低,作用减弱时,退变可发生自主恢复。因此,在临床研究时应注意严格把控干预时间,从而保证实验结果的客观性及准确性。 2.2.5 其他化学制剂 Liu等[35]将N端30 ku Fn-f注入新西兰白兔腰椎间盘的中央区,通过组织学、影像学及蛋白聚糖合成检查椎间盘变化,在为期16周的研究中发现实验组椎间盘中的原始聚糖合成逐渐减少,8周后椎间盘高度下降,12周开始形成明显骨赘,直观呈现出椎间盘退变的过程,与人的自发性退变基本一致。Zhou等[36]采用BrdU作为试验制剂注入羊椎间盘中心区后进行2-14周观察,研究结果表明BrdU不仅可诱导椎间盘退变,而且可制作出优于其他方法的更“生理性”的腰椎间盘退变动物模型。Oda等[37]利用被动吸烟装置进行烟碱诱导小鼠椎间盘退变并获得成功,实验结果提示椎间盘退变可能与吸烟有关。Nemoto等[38]也利用同样的原理制备了鼠吸烟模型,在停止吸烟8周后观察到小鼠椎间盘基质中的纤维链接组织和蛋白聚糖水平有所恢复,但受损的纤维环无法还原,提示戒烟后椎间盘退变在一定程度上有所修复,但有的损伤是不可逆的。Mao等[39]将聚甲基丙烯酸甲酯注射到兔的腰椎间盘中,在手术后3周,椎间盘高度显著降低,另外,与18 G针穿刺模型比,聚甲基丙烯酸甲酯可适度增加椎间盘退变。 化学方式虽能很好地模拟退变椎间盘中蛋白聚糖丢失的典型改变,但是注射的药物有可能影响生物化学检测的准确性,并且注射穿刺的过程本身就是一种损伤建模方法,可能会影响到实验结果的准确性,这些潜在问题及缺陷使其广泛应用受到了一定限制。 "

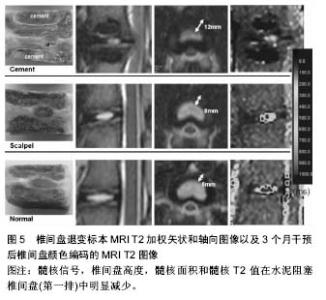
2.3 其他方式 2.3.1 年龄相关 椎体终板软骨细胞的退变是椎间盘退变的始动因素,终板软骨细胞在青年时期后开始出现退变,随着年龄的进一步增加,细胞活力逐渐减弱,凋亡细胞增多,营养代谢失代偿,从而引起软骨终板不可逆性退变。有研究表明软骨细胞凋亡与增龄明显相关,由此可见增龄因素在椎间盘退变中扮演着重要的角色[40]。另外,随着年龄的增长,软骨终板血管芽的相对面积减少,非钙化/钙化比率下降,Ⅱ型胶原含量下降,X型胶原增多。这些表现均提示增龄因素是软骨终板退变的重要影响因素。 Clouet等[41]对3组各年龄层(1,6和30个月)兔的椎间盘进行核磁、组织学分析以及RT-PCR分析髓核细胞中的基因的转录表达。结果验证了兔子腰椎间盘退变程度与年龄的相关性。有学者对1,3,6,12和18月龄大鼠研究发现,大鼠椎间盘结构随月龄增加发生退行性变,多效生长因子及血小板源性生长因子参与了大鼠椎间盘的退变。 此方式简单易行,无需进行繁琐的手术操作,并且实验过程安全,但实验周期长及退变程度不显著是该类造模方式的两大缺陷。 2.3.2 基因修饰技术 随着分子生物水平的提高,改变基因来制造椎间盘退变模型的方式成为可能。Hamrick等[42]去除小鼠氯化筒箭毒碱(GDF)基因后,小鼠的肌肉量与骨密度增加,蛋白多糖含量降低,椎体终板骨化,成功制造小鼠腰椎间盘退变模型。Wu等[43]在调查低氧诱导因子1α的作用时,通过交配产生条件性低氧诱导因子1α敲除(KO)小鼠模型,在Wide型与低氧诱导因子1α KO型两种基因型小鼠的对比研究中发现后者有更多髓核细胞死亡和变性的迹象,并且其椎间盘中聚集蛋白聚糖、Ⅱ型胶原和血管内皮生长因子的表达较Wide型小鼠少,结果表明敲除低氧诱导因子1α的基因型小鼠容易造成椎间盘退变。Bvod等[44]发现缺失Ⅸ型胶原基因的鼠椎间盘病理改变类似人椎间盘退变。Gruber等[45]饲养缺失SPARC基因的小鼠发现纤维环结构紊乱,边界不清。以上研究均提示基因敲除技术制作的退变模型可以用来研究特定蛋白或基因的作用,但基因敲除模型在椎间盘退变研究方面是否能广泛应用,还有待证明。 2.3.3 缺血模型 营养障碍被视为椎间盘退变的原因之一,但在正常椎间盘内尚未发现。Kang等[46]在关于干扰终板营养途径引起猪椎间盘退变的研究中,对实验组猪的双侧终板注射骨水泥诱导供血障碍,3个月后水泥封闭的椎间盘显示严重的椎间盘退变,椎间盘高度指数、髓核面积显著低于正常对照组,此外,经对比得出,水泥阻塞组的椎间盘退变程度超过纤维环穿刺组,详见图5。Wed等[47]建立羊椎间盘营养障碍诱导椎间盘退变模型。切断终板血供并植入钛制薄片以阻止血管再生,证实此种干预措施可严重影响终板的血流灌注,并与对照组相比后抑制因子一氧化氮与此种改变相关。有学者在评价经皮穿刺兔腰椎终板下椎体注射平阳霉素制作椎体终板下缺血模型的可行性时,于实验组椎体内注射平阳霉素,借助平阳霉素干扰血管内皮细胞生长代谢的损伤机制来诱导供血部位缺血,实验结果显示4周时出现明显缺血,5周时出现实验椎体内骨坏死和椎间盘退变。Wei等[48]同样采用平阳霉素行腰椎软骨下骨注射诱导恒河猴腰椎间盘退变。虽然缺血性干预措施的长期作用还有待观察,但它提示了营养障碍在椎间盘退变中起作用的重要信息。此外,缺血模型对于评估新的椎间盘退变和修复的生物学干预措施也有重要意义。"
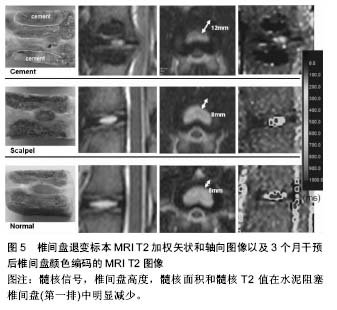
| [1] Sun F, Qu JN, Zhang YG. Animal models of disc degeneration and major genetic strategies. Pain Phys. 2013;16(3): E267-275.[2] 张昭,霍洪军,杨学军,等.兔椎间失稳软骨终板退变的实验研究[J]. 中国矫形外科杂志,2008,16(21):1650-1652.[3] Phillips FM,Reuben J,Wetzel FT.Intervertebral disc degeneration adjacent to a lumbar fusion.Bone Joint Surg[Br]. 2002;84-B:289-294.[4] Zhang K,Ding W.Beta1 integrin inhibits apoptosis induced by cyclic stretch in annulus fibrosus cells via ERK1/2 MAPK pathway. Apopotosis. 2016;21(1):13-24.[5] Lipson SJ,Muir H.1980 Volvo award in basic science Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6:194-210.[6] Sun W, Zhang K, Zhao CQ, et al. Quantitative T2 mapping to characterize the process of intervertebral disc degeneration in a rabbit mode.BMC Musculoskeletal Disorders. 2013;14:357.[7] 崔运能,周荣平,麦奇光,等.肌间隙入路腰椎间盘退变模型的构建[J].南方医科大学学报 2012;32(3):404-408.[8] Obata S, Akeda K, Imanishi T, et al. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res Ther. 2012;14:R241.[9] Mwale F, Masuda K, Pichika R, et al. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration.Arthritis Res Ther.2011;13:R120.[10] Oehme D,Ghosh P.Reconstitution of degenerated ovine lumbar discs by STRO-3-positive allogeneic mesenchymal precursor cells combined with pentosan polysulfate.J Neurosurg Spine. 2016;24(5):715-726.[11] Chen C,Jia Z, Han Z, et al.Quantitative T2 relaxation time and magnetic transfer ratio predict endplate biochemical content ofintervertebral disc degeneration in a canine model.BMC Musculoskelet Disord.2015;16:157.[12] 黄河,李宁宁,胡朝晖,等.纤维环穿刺法与腰椎失稳法建立椎间盘退变模型[J].中国现代医生,2011,49(16):22-24[13] Guehring T, Unglaub F, Lorenz H, et al.Intradiscal pressure measurements in normal discs, compressed discs and compressed discs treated with axial posterior disc distraction: an experimental study on the rabbit lumbar spine model. Eur Spine J. 2006;15:597-604.[14] Guehring T, Nerlich A, Kroeber M, et al.Sensitivity of notochordal disc cells to mechanical loading: an experimental animal.study Eur Spine J. 2010;19:113-121.[15] Lindblom K.Intervertebral-disc degeneration considered as a pressure atrophy.J Bone Joint Surg Am. 1957;39(4):933-945.[16] Wertz K, Godburn K, MacLean JJ, et al. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res. 2009;27(9):1235-1242.[17] Hamilton DJ, Seguin CA, Wang J, et al. Formation of a nucleus pulposus-cartilage endplate construct in vitro. Biomaterials. 2006;27:397-405.[18] Miyamoto S, Yonenobu K, Ono K. Experimental cervical spondylosis in the mouse.Spine (Phila Pa 1976). 1991;16(10 Suppl):S495-500.[19] Shirazi-Adl A, Taheri M, Urban JP. Analysis of cell viability in intervertebral disc: Effect of endplate permeability on cell population. J Biomech. 2010;43(7):1330-1336.[20] Sobajima S,Kompel JF,Kim JS,et al.Aslowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI,X-ray,and histology.Spine.2005;30(1): 15-24.[21] Cinotti G, Della Rocca C, Romeo S, et al.Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine. 2005;30:174-180.[22] Holm S,Holm AK,Ekstrom L,et al. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17(1):64-71.[23] 吕浩然,杨进顺,黄文铎,等.终板损伤建立兔腰椎间盘退变模型[J].广东医学,2008,29(1):44-45.[24] Sato K, Nagata K, Ariyoshi M, et al. Intradiscal pressure after intradiscal injection of hypertonic saline:an experimental study. Eur Spine J. 2000;9:213-217.[25] Hirsch C. Studies on the pathol-ogy of low back pain. Bone Joint Surg Br. 1959;41: 237-243.[26] Smith L.Enzyme dissolution of the nucleus pulposus in humans. Clin Orthop Relat Res. 1986;(206):4-9.[27] Lu DS,Luk KD,Lu WW,et al.Spinal flexibility increase after chymopapain injection is dose dependent:a possible alternative to anterior release in scoliosis.Spine. 2004;29(2): 123-128.[28] 颜登鲁,李健,高梁斌,等.白细胞介素-1β作用下椎间盘退变动物模型的建立[J].实用医学杂志,2006,22(20):2342-2345.[29] Omlor GW,Nerlich AG. A new porcine in vivo animal model of disc degeneration:response of annulus fibrosus cells, chondrocyte-like nucleus pulposus cells,and notochordal nucleuspulposus cells to partial nucleotomy.Spine. 2009; 34(25):2730-2739.[30] Guder E,Hill S,Kandziora F,et al. Partial nucleotomy of the ovine disc as an in vivo model for disc degeneration. Z Orthop Unfall. 2009;147(1):52-58.[31] Hoogendoorn RJ,Helder MN,Kroeze RJ,et al. Reproducible long-term disc degeneration in a large animal model.Spine. 2008;33(9):949-954.[32] Norcross JP,Lester GE,Weinhold P,et al.An in vivo model of degenerative disc disease. J Orthop Res. 2003;21(1):183-188.[33] Peeters M,Detiger SE,et al.BMP-2 and BMP-2/7 Heterodimers Conjugated to a Fibrin/Hyaluronic Acid Hydrogel in a Large Animal Model of Mild Intervertebral Disc Degeneration. Biores Open Access.2015;4(1):398-406.[34] Boxberger JI,Auerbach JD,Sen S,et al.An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine. 2008;33(2):146-154.[35] Liu HF,Zhang H. A novel rabbit disc degeneration model induced by fibronectin fragment. Zhonghua Waike Zazhi. 2013;51(4):362-366.[36] Zhou H,Hou S,Shang W,et al.A new in vivo animal model to create intervertebral disc degeneration characterized by MRI,radiography,CT/discogram,biochemistry,and hostology. Spine. 2007;32(8):864-872.[37] Oda H,Matsuzaki H,Tokuhashi Y,et al.Degeneration of intervertebral discs due to smoking:experimental assessment in a rat-smoking model.J Orthop Sci. 2004;9(2):135-141.[38] Nemoto Y,Matsuzaki H,Tokuhasi Y,et al.Histological changes in intervertebral discs after smoking and cessation: experimental study using a rat passive smoking model.J Orthop Sci. 2006;11(2):191-197.[39] Mao H, Geng D, Zhu X,et al. Intervertebral disc degeneration induced by intradiscal poly(methyl methacrylate) leakage after spine augmentation in an in vivo rabbit model. Acta Biomater. 2014;10(7):3059-3067.[40] Zhang YG, Sun ZM, Liu JT, et al. Features of intervertebraldisc degeneration in rat's aging process. J Zhejiang Univ Sci B. 2009;10(7):522-527.[41] Clouet J, Pot-Vaucel M, Grimandi G, et al. Characterization of the age-dependent intervertebral disc changes in rabbit by correlation between MRI, histology and gene expression.BMC Musculoskelet Disord. 2011;12:1471-1479.[42] Hamrick MW, Pennington C, Byron CD. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF- 8 (myostatin). J Orthop Res. 2003;21(6):1025-1032.[43] Wu WJ, Zhang XK, Zheng XF, et al. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int J Immunopathol Pharmacol.2013;26(3):601-609.[44] Bvod LM, Richardson WJ, Allen KD, et al. Eardy-onest degeneration of the intervertebral disc and vertebral end plate in mice deftcient in typeIX collagen. Arthritis Rheum. 2008; 58(1):164-171.[45] Gruber HE, Sage EH, Norton HJ, et al. Targeted deletion Of the SPARC gene accelerates disc degeneration in the aging mouse.J Histochen Cytochem. 2005;53(9):1131-1138.[46] Kang R, Li H, Ringgaard S, et al.Interference in the endplate nutritional pathway causes intervertebral disc degeneration in an immature porcine model. Int Orthop.2014;38(5):1011-1017.[47] Wed MJ, Lezuo P, Maissen O, et al. Decreased diffusion as a result of perfusion block in the ovine lumbar spine: a future model for disc degeneration.Proceedings of the 52th annual meeting of the orthopaedic research society.The Orthopaedic Research Society, Chicago, USA.2006,1234.[48] Wei F, Zhong R, Wang L, et al. Pingyangmycin-induced in vivo lumbar disc degeneration model of rhesus monkeys. Spine.2015;40(4):E199-210.[49] Zhang Y, Lenart BA, Lee JK, et al. Histological features of endplates of the mammalian spine: from mice to men. Spine. 2014;39(5):E312-317. |
| [1] | Tan Xinfang, Guo Yanxing, Qin Xiaofei, Zhang Binqing, Zhao Dongliang, Pan Kunkun, Li Yuzhuo, Chen Haoyu. Effect of uniaxial fatigue exercise on patellofemoral cartilage injury in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Jiang Huanchang, Zhang Zhaofei, Liang De, Jiang Xiaobing, Yang Xiaodong, Liu Zhixiang. Comparison of advantages between unilateral multidirectional curved and straight vertebroplasty in the treatment of thoracolumbar osteoporotic vertebral compression fracture [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1407-1411. |
| [4] | Wang Baojuan, Zheng Shuguang, Zhang Qi, Li Tianyang. Miao medicine fumigation can delay extracellular matrix destruction in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1180-1186. |
| [5] | Lü Yiyan, Li Hanbing, Ma Xiaoqing, Zhang Han, Zhang Yuhang, Li Genlin. Establishment and characteristic analysis of interior heat and diabetes mouse model using compound factors [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1187-1193. |
| [6] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [7] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [8] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [9] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [10] | Song Jiawei, Yang Yongdong, Yu Xing, Yang Jizhou, Wang Fengxian, Qu Yi, Bi Lianyong. Mid-term effect of Isobar EVO non-fusion dynamic fixation in the treatment of adjacent segment disease after lumbar fusion [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 908-913. |
| [11] | Feng Jianbo, Li Chencheng, Liu Jinyue, Wang Xiaomin, Peng Jiachen. Implantation of Kirschner wire with Staphylococcus aureus biofilm establishes a traumatic osteomyelitis model in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 700-705. |
| [12] | Wang Shihui, Cheng Yang, Zhu Yunjie, Cheng Shaodan, Mao Jianying. Effect of arc edge needle-scalpel therapy on inflammatory factors and histomorphology of the frozen shoulder in rabbit models [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 706-711. |
| [13] | Li Weiming, Xu Qingwen, Li Yijun, Sun Yanbo, Cui Jin, Xu Pengyuan . Deep seawater promotes wound healing in diabetic mice by activating PI3K/Akt pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 724-729. |
| [14] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [15] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||