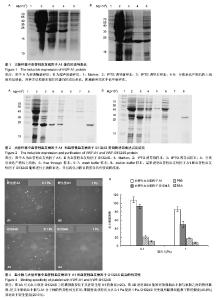
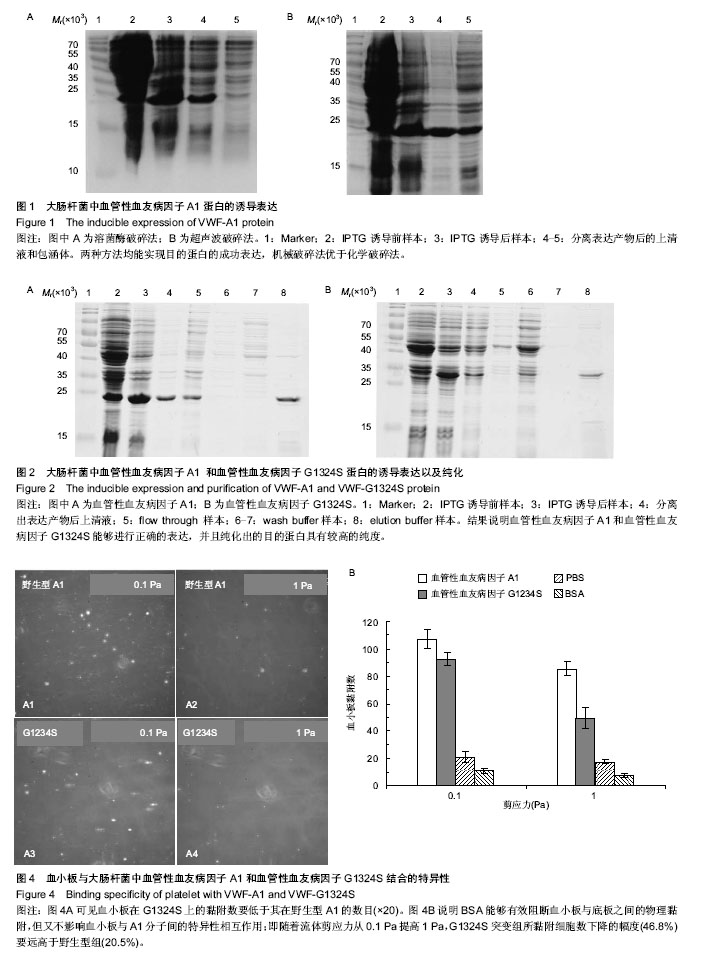
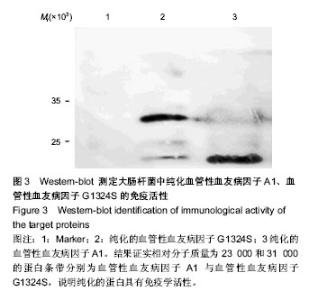
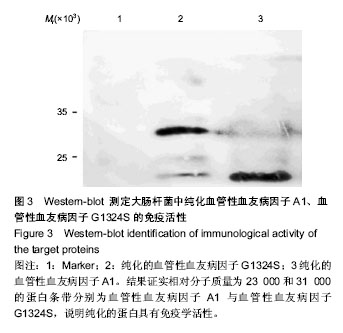
| [1] Savage B, Saldívar E, Ruggeri ZM.Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell.1996;84(2):289-297.
[2] Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost.2006;4(10): 2103-2114.
[3] Schneppenheim R. The pathophysiology of von Willebrand disease: therapeutic implications. Thromb Res.2011;128 Suppl 1: S3-7.
[4] Berber E.The Molecular Genetics of von Willebrand Disease. Turk J Haematol. 2012;29(4):313-324.
[5] Kessler CM.Diagnosis and treatment of von Willebrand disease: new perspectives and nuances. Haemophilia. 2007;13 Suppl 5: 3-14.
[6] Mendolicchio GL, Ruggeri ZM. New perspectives on von Willebrand factor functions in hemostasis and thrombosis. Semin Hematol.2005;42(1): 5-14.
[7] Voorberg J, Fontijn R, van Mourik JA, et al.Domains involved in multimer assembly of von willebrand factor (vWF): multimerization is independent of dimerization[J]. EMBO J.1990;9(3):797-803.
[8] Miura S, Li CQ, Cao Z,et al.Interaction of von Willebrand factor domain A1 with platelet glycoprotein Ibalpha-(1-289). Slow intrinsic binding kinetics mediate rapid platelet adhesion. J Biol Chem.2000;275(11):7539-7546.
[9] De Ceunynck K, De Meyer SF, Vanhoorelbeke K.Vanhoorelbeke. Unwinding the von Willebrand factor strings puzzle. Blood.2013;121(2):270-277.
[10] Cruz MA, Yuan H, Lee JR, et al. Interaction of the von Willebrand factor (vWF) with collagen. Localization of the primary collagen-binding site by analysis of recombinant vWF A domain polypeptides. J Biol Chem.1995;270(33): 19668.
[11] Schulteam Esch J 2nd, Cruz MA, Siegel JB, et al. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood.1997;90(11): 4425-4437.
[12] Li Z, Delaney MK, O'Brien KA,et al.Signaling during platelet adhesion and activation[J]. Arterioscler Thromb Vasc Biol. 2010;30(12): 2341-2349.
[13] Berntorp E. Von Willebrand disease.Pediatric blood & cancer, 2013. 60(S1):S34-S36.
[14] Cruz MA, Diacovo TG, Emsley J, et al.Mapping the glycoprotein Ib-binding site in the von willebrand factor A1 domain.J Biol Chem.2000;275(25): 19098-19105.
[15] Goodeve AC. The genetic basis of von Willebrand disease. Blood Rev.2010; 24(3):123-134.
[16] Emsley J, Cruz M, Handin R,et al.Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J Biol Chem. 1998;273 (17):10396-10401.
[17] Morales LD, Martin C, Cruz MA.The interaction of von Willebrand factor-A1 domain with collagen: mutation G1324S (type 2M von Willebrand disease) impairs the conformational change in A1 domain induced by collagen. J Thromb Haemost. 2006;4(2): 417-425.
[18] Liu G, Fang Y, Wu J. A mechanism for localized dynamics-driven affinity regulation of the binding of von Willebrand factor to platelet glycoprotein Ibalpha. J Biol Chem.2013;288(37):26658-26667.
[19] Ju L, Dong JF, Cruz MA, et al. The N-terminal Flanking Region of the A1 Domain Regulates the Force-dependent Binding of von Willebrand Factor to Platelet Glycoprotein Ibalpha. J Biol Chem.2013;288(45):32289-32301.
[20] Gardiner EE, Arthur JF, Shen Y, et al. GPIbalpha-selective activation of platelets induces platelet signaling events comparable to GPVI activation events. Platelets.2010;21(4): 244-252.
[21] Young CL, Britton ZT, Robinson AS.Recombinant protein expression and purification: a comprehensive review of affinity tags and microbial applications. Biotechnol J. 2012; 7(5): 620-634.
[22] Li Q, Fang Y, Ding X, et al. Force-dependent bond dissociation govern rolling of HL-60 cells through E-selectin. Experimental cell research.2012;318(14):1649-1658.
[23] Coburn LA,Damaraju VS,Dozic S,et al.GPIbalpha-vWF rolling under shear stress shows differences between type 2B and 2M von Willebrand disease. Biophys J.2011;100(2): 304-312.
[24] Ulrichts H, Udvardy M, Lenting PJ, et al. Shielding of the A1 domain by the D'D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V.J Biol Chem.2006;281(8): 4699-4707.
[25] Cruz MA, Handin RI, Wise RJ. The interaction of the von Willebrand factor-A1 domain with platelet glycoprotein Ib/IX. The role of glycosylation and disulfide bonding in a monomeric recombinant A1 domain protein. J Biol Chem. 1993;268(28):21238-21245.
[26] Yago T, Lou J, Wu T, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest.2008;118(9): 3195-3207. |