Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (16): 2874-2882.doi: 10.3969/j.issn.2095-4344.2013.16.004
Previous Articles Next Articles
Safety of true bone ceramics
Liu Bin-yu1, Guo Min-fang1, Xing Yan-xia1, Ma Cun-gen2, 3.
- 1 Medical School, Shanxi Datong University, Datong 037009, Shanxi Province, China
2 Institute of Brain Science, Shanxi Datong University, Datong 037009, Shanxi Province, China
3 Shanxi University of Traditional Chinese Medicine, Taiyuan 030001, Shanxi Province, China
-
Online:2013-04-16Published:2013-04-16 -
Contact:Ma Cun-gen, Professor, Doctoral supervisor, Institute of Brain Science, Shanxi Datong University, Datong 037009, Shanxi Province, China; Shanxi University of Traditional Chinese Medicine, Taiyuan 030001, Shanxi Province, China -
About author:Liu Bin-yu★, Master, Associate professor, Medical School, Shanxi Datong University, Datong 037009, Shanxi Province, China liudaifu775@163.com -
Supported by:Shanxi University Technology Research and Development Projects, No. 20111120*
CLC Number:
Cite this article
Liu BY, Guo MF, Xing YX, Ma CG. Safety of true bone ceramics[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(16): 2874-2882.
share this article

Appearance shape of true bone ceramic Bovine cancellous bone was white (Figure 1). The special fluorescent character of hydroxyapatite was found. The trabecular pattern presented the full structure, and the trabecular gap was the interconnected pore. The three-dimensional mesh structure was observed by electron microscope and the pore diameter was unequal 190-750 μm, the porosity was (80.39±1.40)%. On one hand, the connective mesh structure will benefit to the formation of osteon, which could provide larger surface for the proliferation of osteoblasts. On the other hand, it will benefit to the infiltration of nutritional ingredient and formation of vascular and degradation of materials. Morphology of true bone ceramic-bone marrow mesenchymal stem cells complex"

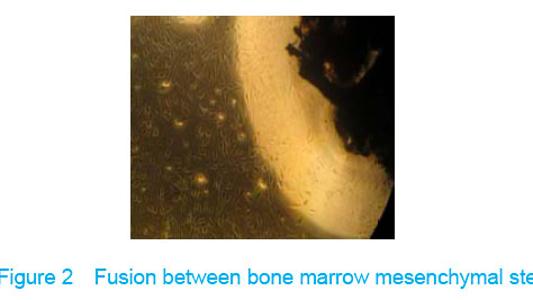
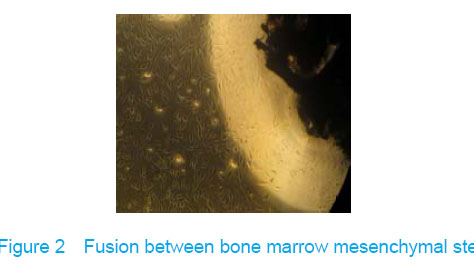
Observation under invert microscope True bone ceramic mesh was filled with bone marrow mesenchymal stem cells immediately after bone marrow mesenchymal stem cells inoculated into the true bone ceramic, lots of cells adhered to the bracket after observed for 24 hours, and large extracellular matrix was secreted after 7 days. There was no clear boundary between the cells and the matrix, which indicated that there had fine fusion between the cell and the true bone ceramic (Figure 2)."
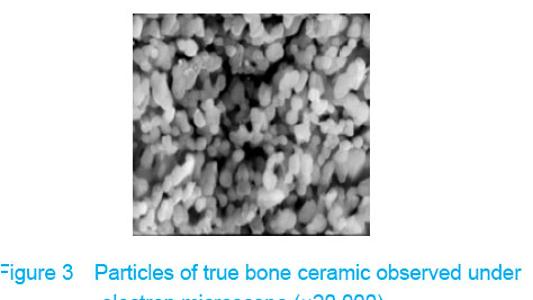
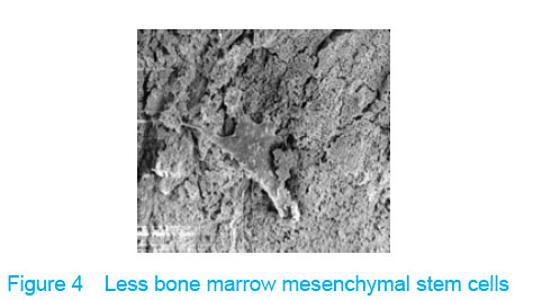
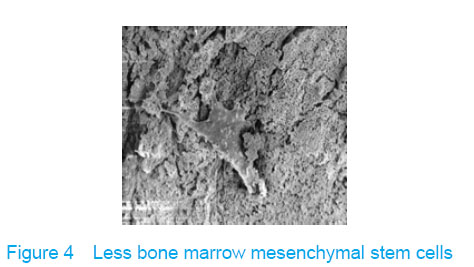
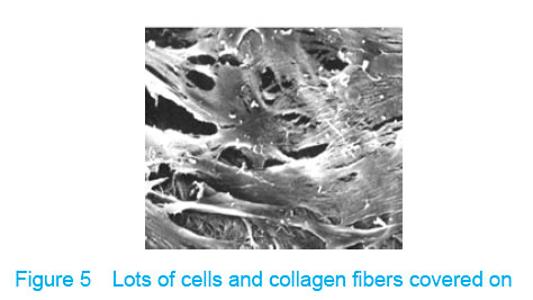
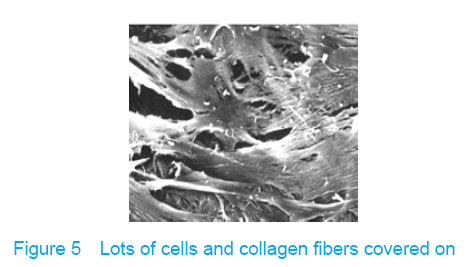
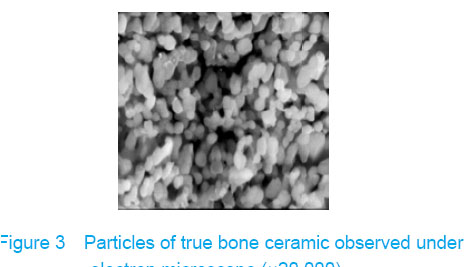
Observation with scanning electron microscope Electron microscope showed that a few cells adhered to true bone ceramic at 1 day after bone marrow mesenchymal stem cells incubation, and lots of cells and collagen fibers covered on the scaffold at 7 days and embedded scaffold basically (Figures 3-5)."
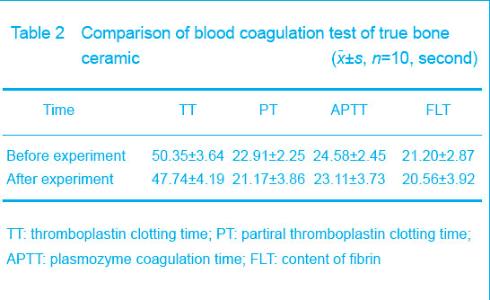
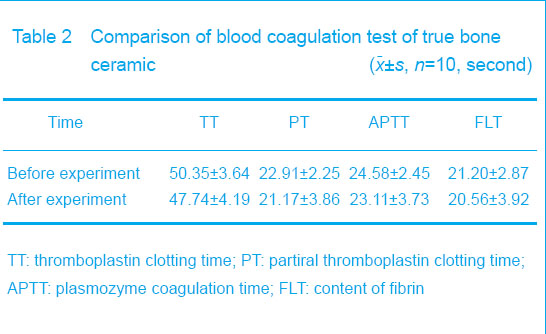
Blood coagulation test Blood coagulation test showed that there were no changes in plasmozyme coagulation time, partiral thromboplastin clotting time, thromboplastin clotting time and the content of fibrin before and after the experiment (P > 0.05), which indicated that the materials could not change the coagulation function (Table 2)."
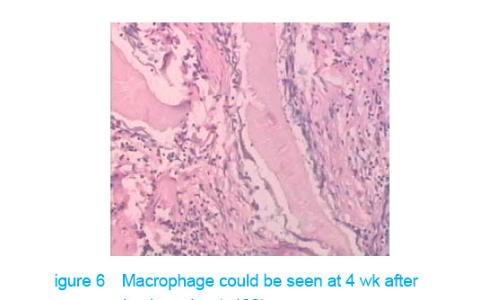
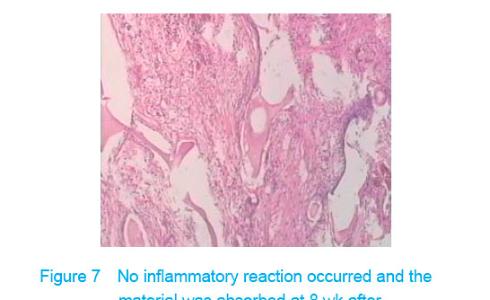
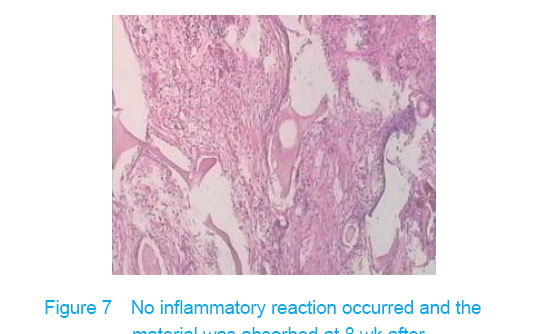
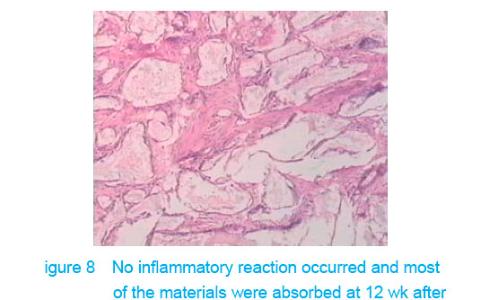
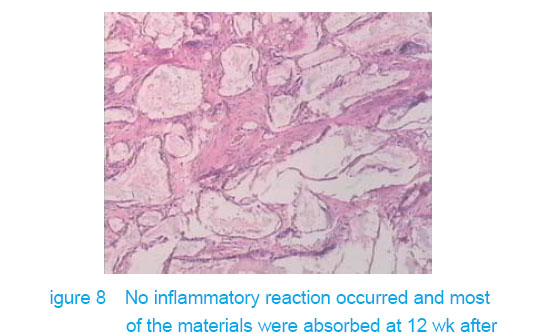
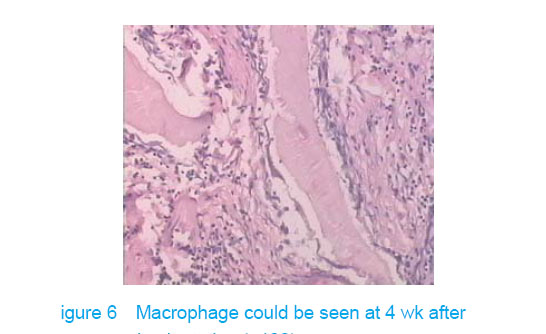
Test of subcutaneous implantation There was no wound infection, suppuration and rejection after subcutaneous implantation. Muscle color and texture was normal around material. At 4 weeks, there had slight inflammatory cell infiltration around the implantation and there had macrophage. Fibrous tissue grew to the interior of material and the fibers arranged disorderly. At 8 weeks, the morphology of muscle tissue was normal around materials, regular collagen fibers appeared and there was no imflammatory cell infiltration. At 12 weeks, part of the materials degraded and were replaced by newly developed fibrous tissue (Figures 6-8)."
| [1]Liu BY, Ma XH, Li NY, et al. Biocompatibility and cytocompatibility of calcined Bone. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(41): 8055-8058.[2]Qiao GY, Su JS. Advances of composite scaffold materials for bone tissue engineering. Kouqiang Hemian Waike Zazhi. 2010;20(5):374-377.[3]Deligianni DD, Katsala ND, Koutsoukos PG, et al. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22(1):87-96.[4]Liu BY, Li NY, Fan GW, et al. Experimental study of cultivation, identification and induced differentiation of bone marrow stromal stem cells into osteoblasts in rats. Qilu Yixue Zazhi. 2007;22(3):215-217.[5]The Ministry of Science and Technology of the People′s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.[6]Liu BY, Li NY, Fan GW, et al. In vitro osteoblastic differentiation and identification of rat bone marrow mesenchymal stem cells by whole bone marrow adherent culture. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2007;11(50):10181-10184.[7]Zang HM, Liu YH, Chen JC, et al. An experimental research on different temperature sintered bone as carrier of bone morphogenetic protein. Shengwu Yixue Gongchengxue Zazhi. 2006;23(2):366-369.[8]Yang CL, Bi ZG, Wang YX, et al. The osteoblast differentiation of rabbit bone marrow stromal cells and construction with true bone ceramic. Zhongguo Guzhi Shusong Zazhi. 2005;11(3):329-332.[9]Lu W, Tao K, Mao TQ, et al. Experimental study of marrow stromal osteoMasts culture with caldned bone caldam. Zhongguo Linchuang Kangfu. 2003;7(2):214-215.[10]Zhang DZ, Fan QY, Ma BA, et al. Assessment on biological safety of material combined with true bone ceramic, bBMP and bone cement. Zhongguo Linchuang Kangfu. 2002;6(14):2060-2061.[11]Xu YH, Shi XY, Hu YY, et al. Osteogenetic activity in vivo of true bone ceramic with osteoblastic compound substances. Disi Junyi Daxue Xuebao. 2002;23(3):223-226.[12]Zheng QX, Liu SN. The preparation of sintered bovine cancellous bone and a study of its mechanical and chemical behavior and biocompatibility. Shengwu Yixue Gongchengxue Zazhi. 2005;22(1):95-98.[13]Li LF. Drug Experiment and Preparation. Beijing: University of Science and Technology of China Press. 2010:61,67,69.[14]Du Y, Ke ZY. Decalcified bone matrix and bone cement compound at various proportions in repairing rabbit femoral defect. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2009;13(21):4064-4068.[15]Wingerter S, Tucci M, Bumgardner J, et al. Evaluation of short-term healing following sustained delivery of osteoinductive agents in a rat femur drill defect model. Biomed Sci Instrum. 2007;43:188-193.[16]Cool SM, Kenny B, Wu A, et al. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)composite biomaterials for bone tissue regeneration: in vitro performance assessed by osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J Biomed Mater Res A. 2007;82(3):599-610.[17]Leivo J, Meretoja V, Vippola M, et al. Sol-gel derived aluminosilicate coatings on alumina as substrate for osteoblasts. Acta Biomater. 2006;2(6):659-668.[18]Le Nihouannen D, Saffarzadeh A, Gauthier O, et al. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med. 2008;19(2):667-675.[19]Yan X, Huang X, Yu C, et al. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials. 2006;27(18): 3396-3403.[20]Chen LQ, Li NY, Yuan RT, et al. Study of MSCs in vitro cultured on demineralized bone matrix of mongrel. Shanghai Kouqiang Yixue. 2007;16(3):255-258.[21]Li NY, Chen LQ, Chen T, et al. Effect of platelet-rich plasma and latissimus dorsi myofascia with blood vessel on vascularization of tissue engineered bone in dogs. Huaxi Kouqiang Yixue Zazhi. 2007;25(4):408-411. |
| [1] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [2] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [5] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [6] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [7] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [8] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [9] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [10] | Wang Haiying, Lü Bing, Li Hui, Wang Shunyi. Posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis: prediction of functional prognosis of patients based on spinopelvic parameters [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1393-1397. |
| [11] | Lü Zhen, Bai Jinzhu. A prospective study on the application of staged lumbar motion chain rehabilitation based on McKenzie’s technique after lumbar percutaneous transforaminal endoscopic discectomy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1398-1403. |
| [12] | Chen Xinmin, Li Wenbiao, Xiong Kaikai, Xiong Xiaoyan, Zheng Liqin, Li Musheng, Zheng Yongze, Lin Ziling. Type A3.3 femoral intertrochanteric fracture with augmented proximal femoral nail anti-rotation in the elderly: finite element analysis of the optimal amount of bone cement [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1404-1409. |
| [13] | Du Xiupeng, Yang Zhaohui. Effect of degree of initial deformity of impacted femoral neck fractures under 65 years of age on femoral neck shortening [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1410-1416. |
| [14] | Zhang Shangpu, Ju Xiaodong, Song Hengyi, Dong Zhi, Wang Chen, Sun Guodong. Arthroscopic suture bridge technique with suture anchor in the treatment of acromioclavicular dislocation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1417-1422. |
| [15] | Liang Yan, Zhao Yongfei, Xu Shuai, Zhu Zhenqi, Wang Kaifeng, Liu Haiying, Mao Keya. Imaging evaluation of short-segment fixation and fusion for degenerative lumbar scoliosis assisted by highly selective nerve root block [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1423-1427. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||